Protective immunity to nematode infection is induced by CTLA-4 blockade - PubMed (original) (raw)
Protective immunity to nematode infection is induced by CTLA-4 blockade
K McCoy et al. J Exp Med. 1997.
Abstract
The recent observation that neutralization or genetic deletion of the T lymphocyte receptor CTLA-4 allows enhanced T cell reactivity offers new opportunities for immunotherapy against infectious agents. We used a neutralizing antibody to block CTLA-4 interaction with its ligands CD80 and CD86 during infection of mice with the nematode, Nippostrongylus brasiliensis. CTLA-4 blockade greatly enhanced and accelerated the T cell immune response to N. brasiliensis, resulting in a profound reduction in adult worm numbers and early termination of parasite egg production. The ability of CTLA-4 blockade to accelerate primary immune responses to a protective level during an acute infection indicates its potential as an immunotherapeutic tool for dealing with infectious agents.
Figures
Figure 1
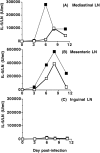
Treatment with anti–CTLA-4 greatly enhances IL-5 production in lymph nodes draining the sites of infection with Nb. C57BL/6 mice were inoculated i.p. with 1,000 Nb L3 larvae and treated with control hamster IgG (open squares) or hamster anti–mouse CTLA-4 mAb (filled squares) at 1 mg/week beginning at day 0. Total lymph node cells were stimulated in vitro with anti-CD3 and IL-5 levels in the supernatant measured by ELISA. Values represent the mean concentration of IL-5 produced from 1 × 106 cells from 4 mice and adjusted to IL-5 production per lymph node based on the total lymph node cell number. Results shown are representative of three separate experiments. (A) IL-5 production from the draining mediastinal lymph node. (B) IL-5 production from the draining mesenteric lymph node. (C) IL-5 production from the inguinal lymph node. This lymph node does not drain any site of Nb infection.
Figure 2
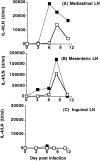
Treatment with anti–CTLA-4 greatly enhances IL-4 production in lymph nodes draining the sites of infection with Nb. C57BL/6 mice were inoculated i.p. with 1,000 Nb L3 larvae and treated with control hamster IgG (open squares) or hamster anti–mouse CTLA-4 mAb (filled squares) as in Fig. 1. Total lymph node cells were stimulated in vitro as described in Fig. 1 and IL-4 levels in the supernatant measured by ELISA. Values represent the mean concentration of IL-4 produced from 1 × 106 cells from four mice and adjusted to IL-4 production per lymph node based on the total lymph node cell number. Results shown are representative of three separate experiments. (A) IL-4 production from the draining mediastinal lymph node. (B) IL-4 production from the draining mesenteric lymph node. (C) IL-4 production from the inguinal lymph node. This lymph node does not drain any site of Nb infection.
Figure 3
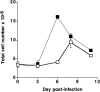
Treatment with anti–CTLA-4 increases total lymphocyte numbers in mediastinal lymph nodes of Nb-infected mice. C57BL/6 mice were inoculated i.p. with 1,000 Nb L3 larvae and treated with control hamster IgG (open squares) or hamster anti–mouse CTLA-4 mAb (filled squares) as in Fig. 1. Values represent the mean cell number from four mice ± SE and results are representative of three separate experiments.
Figure 4
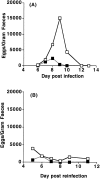
Treatment with anti–CTLA-4 decreases parasite egg output in a primary Nb infection and does not affect development of memory to a subsequent infection with Nb. (A) C57BL/6 mice were inoculated i.p. with 1,000 L3 Nb larvae and left untreated (open squares) or treated with anti–CTLA-4 neutralizing mAb (filled squares) as described in Fig. 1. Feces were collected daily from groups of mice (n = 8) starting day 6 after infection. (B) Mice from A were inoculated i.p. with 1,000 L3 Nb larvae 68 d after primary Nb infection. During the primary infection mice were untreated (open squares) or treated with anti–CTLA-4 mAb (filled squares) as above. The last injection of mAb was given 21 d after primary infection. Feces were collected daily from groups of mice (n = 8) starting day 5 after challenge.
Similar articles
- CTLA4-Ig inhibits optimal T helper 2 cell development but not protective immunity or memory response to Nippostrongylus brasiliensis.
Harris NL, Peach RJ, Ronchese F. Harris NL, et al. Eur J Immunol. 1999 Jan;29(1):311-6. doi: 10.1002/(SICI)1521-4141(199901)29:01<311::AID-IMMU311>3.0.CO;2-B. Eur J Immunol. 1999. PMID: 9933113 - Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo.
Liu Z, Liu Q, Pesce J, Whitmire J, Ekkens MJ, Foster A, VanNoy J, Sharpe AH, Urban JF Jr, Gause WC. Liu Z, et al. J Immunol. 2002 Dec 15;169(12):6959-68. doi: 10.4049/jimmunol.169.12.6959. J Immunol. 2002. PMID: 12471130 - CTLA-4 ligands are required to induce an in vivo interleukin 4 response to a gastrointestinal nematode parasite.
Lu P, Zhou X, Chen SJ, Moorman M, Morris SC, Finkelman FD, Linsley P, Urban JF, Gause WC. Lu P, et al. J Exp Med. 1994 Aug 1;180(2):693-8. doi: 10.1084/jem.180.2.693. J Exp Med. 1994. PMID: 8046343 Free PMC article. - The development of CD4+ T effector cells during the type 2 immune response.
Gause WC, Ekkens M, Nguyen D, Mitro V, Liu Q, Finkelman FD, Greenwald RJ, Urban JF. Gause WC, et al. Immunol Res. 1999;20(1):55-65. doi: 10.1007/BF02786507. Immunol Res. 1999. PMID: 10467983 Review. - Role of B7 signaling in the differentiation of naive CD4+ T cells to effector interleukin-4-producing T helper cells.
Gause WC, Urban JF, Linsley P, Lu P. Gause WC, et al. Immunol Res. 1995;14(3):176-88. doi: 10.1007/BF02918215. Immunol Res. 1995. PMID: 8778208 Review.
Cited by
- Acute Malaria Induces PD1+CTLA4+ Effector T Cells with Cell-Extrinsic Suppressor Function.
Mackroth MS, Abel A, Steeg C, Schulze Zur Wiesch J, Jacobs T. Mackroth MS, et al. PLoS Pathog. 2016 Nov 1;12(11):e1005909. doi: 10.1371/journal.ppat.1005909. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27802341 Free PMC article. - Pinpointing when T cell costimulatory receptor CTLA-4 must be engaged to dampen diabetogenic T cells.
Luhder F, Chambers C, Allison JP, Benoist C, Mathis D. Luhder F, et al. Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):12204-9. doi: 10.1073/pnas.200348397. Proc Natl Acad Sci U S A. 2000. PMID: 11035773 Free PMC article. - Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection.
Johanns TM, Ertelt JM, Rowe JH, Way SS. Johanns TM, et al. PLoS Pathog. 2010 Aug 12;6(8):e1001043. doi: 10.1371/journal.ppat.1001043. PLoS Pathog. 2010. PMID: 20714351 Free PMC article. - Helminthic therapy: improving mucosal barrier function.
Wolff MJ, Broadhurst MJ, Loke P. Wolff MJ, et al. Trends Parasitol. 2012 May;28(5):187-94. doi: 10.1016/j.pt.2012.02.008. Epub 2012 Mar 28. Trends Parasitol. 2012. PMID: 22464690 Free PMC article. Review. - Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy.
Borkow G, Bentwich Z. Borkow G, et al. Clin Microbiol Rev. 2004 Oct;17(4):1012-30, table of contents. doi: 10.1128/CMR.17.4.1012-1030.2004. Clin Microbiol Rev. 2004. PMID: 15489359 Free PMC article. Review.
References
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources