Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy - PubMed (original) (raw)
Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy
M Wick et al. J Exp Med. 1997.
Abstract
One enigma in tumor immunology is why animals bearing malignant grafts can reject normal grafts that express the same nonself-antigen. An explanation for this phenomenon could be that different T cell clones react to the normal graft and the malignant cells, respectively, and only the tumor-reactive clonotypes may be affected by the growing tumor. To test this hypothesis, we used a T cell receptor transgenic mouse in which essentially all CD8(+) T cells are specific for a closely related set of self-peptides presented on the MHC class I molecule Ld. We find that the tumor expressed Ld in the T cell receptor transgenic mice but grew, while the Ld-positive skin was rejected. Thus, despite an abundance of antigen-specific T cells, the malignant tissue grew while normal tissue expressing the same epitopes was rejected. Therefore, systemic T cell exhaustion or anergy was not responsible for the growth of the antigenic cancer cells. Expression of costimulatory molecules on the tumor cells after transfection and preimmunization by full-thickness skin grafts was required for rejection of a subsequent tumor challenge, but there was no detectable effect of active immunization once the tumor was established. Thus, the failure of established tumors to attract and activate tumor-specific T cells at the tumor site may be a major obstacle for preventive or therapeutic vaccination against antigenic cancer.
Figures
Figure 1
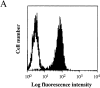
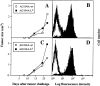
Homogenous expression of the Ld molecule on the transfected tumor cells and of the anti-Ld TCR 2C on the transgenic CD8+ T cells. (A) Stable transfection of AG104A tumor cells with an Ld cDNA expression vector resulted in Ld expression 40-fold above background as shown by FACS® analysis. Shaded curve, anti-Ld staining; unshaded curve, staining with goat anti–mouse FITC secondary antibody alone. (B) Two-color staining of peripheral blood cells from the C3H × 2C transgenic mice with biotinylated anti-CD8a, and 1B2–FITC (anti–2C) mAbs showed that all CD8+ T cells express the 2C TCR.
Figure 1
Homogenous expression of the Ld molecule on the transfected tumor cells and of the anti-Ld TCR 2C on the transgenic CD8+ T cells. (A) Stable transfection of AG104A tumor cells with an Ld cDNA expression vector resulted in Ld expression 40-fold above background as shown by FACS® analysis. Shaded curve, anti-Ld staining; unshaded curve, staining with goat anti–mouse FITC secondary antibody alone. (B) Two-color staining of peripheral blood cells from the C3H × 2C transgenic mice with biotinylated anti-CD8a, and 1B2–FITC (anti–2C) mAbs showed that all CD8+ T cells express the 2C TCR.
Figure 2
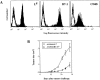
AG104A–Ld tumor cells grow progressively and retain Ld expression in normal C3H × C57BL/6 mice and anti-Ld TCR transgenic mice. AG104A–Ld cells were injected subcutaneously into the flanks of four normal C3H × C57BL/6 mice and five anti-Ld C3H × 2C TCR transgenic mice (A) or two nude mice and three anti-Ld C3H × 2C TCR transgenic mice (B). Tumors grew out in all types of mice. Bars indicate the SEM. FACS® analysis of AG104A–Ld tumor cells reisolated at day 27 from a nontransgenic mouse (C) or at day 33 from a TCR transgenic mouse (D) showed that antigen expression was retained in the course of tumor growth. Shaded curve, anti-Ld staining; unshaded curve, staining with goat anti–mouse FITC secondary antibody alone.
Figure 3
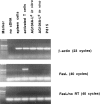
AG104A–Ld tumor cells do not express Fas ligand. Total RNA was isolated from AG104A–Ld tumor cells grown in culture or as tumors in vivo, P815 tumor cells, and spleen cells. Total RNA from the T cell clone pGL10 (37), activated for 7 d on syngeneic feeder cells in the presence of 0.2 mg/ml chicken ovalbumin and 12 IU/ml rhuIL-2, was provided by Dr. U. Korthäuer (University of Chicago). Expression of Fas ligand and β-actin was analyzed by RT-PCR. To test for genomic DNA contamination, RT-PCR was also performed without the addition of reverse transcriptase for the cDNA synthesis (no RT).
Figure 4
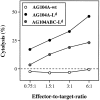
An anti-Ld–specific CD8+ T cell clone from TCR transgenic mice has cytolytic activity against AG104A–Ld and AG104ABC–Ld tumor cells in vitro. The CD8+ T cell clone 900-2 derived from the 2C TCR transgenic mice specifically recognizes and lyses AG104A–Ld and AG104ABC–Ld tumor cells, but not AG104A–wt tumor cells in a 4.5-h 51Cr release assay in vitro.
Figure 5
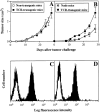
Anti-Ld TCR transgenic mice reject Ld-positive skin, but do not reject a simultaneous challenge with Ld-expressing tumor cells. Two TCR transgenic mice were transplanted with full-thickness Ld-positive skin from a BALB/c mouse and concurrently received subcutaneous injections of AG104A–wt cells and AG104A–Ld cells on opposite flanks. Whereas both mice rejected the skin graft at day 13 after transplantation (see Table 1), both wt and Ld-expressing tumors grew progressively (A and C). Bars indicate the SEM. The outgrowth of AG104A–Ld was not due to antigen loss, because tumor cells reisolated on day 24 still stained positive for Ld in a FACS® analysis (B and D). Shaded curve, anti-Ld staining, unshaded curve, staining with goat anti–mouse FITC secondary antibody alone.
Figure 6
Expression of the costimulatory molecules B7-1 and CD48 by the Ld-positive AG104A tumor cells leads to slower tumor outgrowth in naive TCR transgenic mice. AG104A cells expressing the costimulatory molecules B7-1 and CD48 (18) were transfected with an Ld cDNA expression vector. The expression level of Ld, B7-1, and CD48 in these triple transfected cells, named AG104ABC–Ld, is shown in A. To determine the effect of expression of B7-1 and CD48 on tumor cell outgrowth (B), naive TCR transgenic mice were injected with either AG104A–Ld cells (3 mice) or AG104ABC–Ld cells (11 mice). AG104ABC–Ld tumor cells grew slower than AG104A–Ld tumor cells. Bars indicate the SEM.
Figure 7
Antigen-positive tumor cells providing costimulation are effectively rejected after preimmunization of TCR transgenic mice with antigen-positive skin. Six TCR transgenic mice were challenged subcutaneously with AG104ABC–Ld tumor cells and AG104ABC tumor cells on opposite flanks 3–5 d after rejection of a full-thickness BALB/c skin graft. All six mice rejected the AG104ABC–Ld tumor cells, but not the simultaneous AG104ABC tumor cell challenge, showing that the protective effect of Ld-positive skin was antigen specific (A). Four mice, each of which rejected the skin graft, were treated with anti-CD8 antibody (B) or anti-CD4 antibody (C) 3 d before challenge with AG104ABC–Ld tumor cells. The tumors grew in the anti-CD8–treated mice, but not in the anti-CD4–treated mice, showing that tumor rejection is mediated by CD8+ T cells. Spleen cells from the six mice that rejected the AG104ABC–Ld tumor cells (D) and six naive TCR transgenic mice (E) were stimulated in vitro with AG104A–Ld tumor cells in a MLTC. Specific cytolytic T cells were detected only in the culture from mice that had rejected the AG104ABC–Ld tumor challenge. Bars indicate the SEM.
Similar articles
- Animals bearing malignant grafts reject normal grafts that express through gene transfer the same antigen.
Perdrizet GA, Ross SR, Stauss HJ, Singh S, Koeppen H, Schreiber H. Perdrizet GA, et al. J Exp Med. 1990 Apr 1;171(4):1205-20. doi: 10.1084/jem.171.4.1205. J Exp Med. 1990. PMID: 2324687 Free PMC article. - Natural killer cells recognize common antigenic motifs shared by H-2Dd, H-2Ld and possibly H-2Dr molecules expressed on bone marrow cells.
Yu YY, Forman J, Aldrich C, Blazar B, Flaherty L, Kumar V, Bennett M. Yu YY, et al. Int Immunol. 1994 Sep;6(9):1297-306. doi: 10.1093/intimm/6.9.1297. Int Immunol. 1994. PMID: 7529556 - Autoaggression and tumor rejection: it takes more than self-specific T-cell activation.
Ganss R, Limmer A, Sacher T, Arnold B, Hämmerling GJ. Ganss R, et al. Immunol Rev. 1999 Jun;169:263-72. doi: 10.1111/j.1600-065x.1999.tb01321.x. Immunol Rev. 1999. PMID: 10450523 Review. - Comparative study of the role of professional versus semiprofessional or nonprofessional antigen presenting cells in the rejection of vascularized organ allografts.
Sundstrom JB, Ansari AA. Sundstrom JB, et al. Transpl Immunol. 1995 Dec;3(4):273-89. doi: 10.1016/0966-3274(95)80013-1. Transpl Immunol. 1995. PMID: 8665146 Review.
Cited by
- Immune selection in neoplasia: towards a microevolutionary model of cancer development.
Pettit SJ, Seymour K, O'Flaherty E, Kirby JA. Pettit SJ, et al. Br J Cancer. 2000 Jun;82(12):1900-6. doi: 10.1054/bjoc.2000.1206. Br J Cancer. 2000. PMID: 10864195 Free PMC article. Review. - Immunotherapy in gastric cancer.
Matsueda S, Graham DY. Matsueda S, et al. World J Gastroenterol. 2014 Feb 21;20(7):1657-66. doi: 10.3748/wjg.v20.i7.1657. World J Gastroenterol. 2014. PMID: 24587645 Free PMC article. Review. - Dendritic cells as adjuvants for immune-mediated resistance to tumors.
Schuler G, Steinman RM. Schuler G, et al. J Exp Med. 1997 Oct 20;186(8):1183-7. doi: 10.1084/jem.186.8.1183. J Exp Med. 1997. PMID: 9379142 Free PMC article. Review. No abstract available. - Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells.
Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Overwijk WW, et al. J Exp Med. 2003 Aug 18;198(4):569-80. doi: 10.1084/jem.20030590. J Exp Med. 2003. PMID: 12925674 Free PMC article. - Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication.
Quezada SA, Peggs KS, Simpson TR, Allison JP. Quezada SA, et al. Immunol Rev. 2011 May;241(1):104-18. doi: 10.1111/j.1600-065X.2011.01007.x. Immunol Rev. 2011. PMID: 21488893 Free PMC article. Review.
References
- Chen L, Linsley PS, Hellström KE. Costimulation of T cells for tumor immunity. Immunol Today. 1993;14:483–486. - PubMed
- Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/FAS) ligand-expressing tumor cells—a mechanism of immune evasion? . Nature Med. 1996;2:1361–1370. - PubMed
- Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science (Wash DC) 1996;274:1363–1366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA022677/CA/NCI NIH HHS/United States
- R01-CA22677/CA/NCI NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- CA-14599/CA/NCI NIH HHS/United States
- R01 CA037156/CA/NCI NIH HHS/United States
- R01-CA37156/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous