Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum - PubMed (original) (raw)
Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum
S J Cragg et al. J Neurosci. 1997.
Abstract
Dopamine (DA) is released from somatodendritic sites of neurons in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA), where it has neuromodulatory effects. The aim of this study was to evaluate the role of D2 autoreceptor inhibition in the regulation of this somatodendritic release in each region. Fast cyclic voltammetry at carbon fiber microelectrodes was used to measure electrically evoked DA release in vitro. Furthermore, we compared D2 regulation of somatodendritic release with the more familiar axon terminal release in caudate putamen (CPu) and nucleus accumbens (NAc). Evoked DA release was TTX-sensitive at all sites. There was significant D2 autoinhibition of DA release in SNc; however, this mechanism was two- to threefold less powerful, as compared with axon terminal release in CPu. In contrast to SNc, somatodendritic release in VTA was not under significant D2 receptor control, whereas release in the respective axon terminal region (NAc) was controlled strongly by autoinhibition. Thus, these data indicate that, first, autoinhibition via D2 receptors consistently plays a less significant role in the control of somatodendritic than axon terminal DA release, and, second, even at the level of somatodendrites themselves, D2 autoinhibition displays marked regional variation. In the light of previous data indicating that DA uptake processes are also less active in somatodendritic than in terminal regions, these results are interpreted as indicating that DA transmission is regulated differently in somatodendritic zones, as compared with axon terminals, and thus may have different functional consequences.
Figures
Fig. 3.
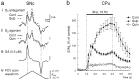
Typical voltammograms and traces of the effect of D2 receptor modulation on [DA]o evoked by a 10 Hz sustained stimulation in SNc and_CPu_. a, Typical voltammograms of maximum [DA]o obtained during stimulation (10 Hz, 40 pulses) in_SNc_ in control (Cont, solid line) and with (i) sulpiride (Sulp, 1 μ
m
) or (ii) quinpirole (Quin, 1 μ
m
; dashed traces). DA oxidation current was increased in the presence of sulpiride and unaffected by the application of quinpirole. The peak oxidation and reduction potentials of DA are indicated by (iii) a DA calibration voltammogram in Ringer’s solution at 32°C and occur at +530 and −180 mV, respectively, versus Ag/AgCl (dotted lines). The voltammograms are scaled to illustrate relative concentrations. Electrode sensitivity to DA was 6.0–16.4 nA/μ
m
. Scale bars: 1 nA and 1 msec.iv, The applied FCV voltage waveform versus Ag/AgCl.b, Averaged traces of evoked [DA]o versus time during stimulation (10 Hz, 30 pulses) in CPu. Stimulation was from t = 1.0 to_t_ = 4.0 sec (solid bar). In control (•), [DA]o was maximal at 0.5 ± 0.25 sec after the start of the stimulus after a fast rise phase and then declined during stimulation. In the presence of D2 antagonism by sulpiride (▴), the decline phase of [DA]o was eliminated and [DA]o increased throughout the stimulus duration, reaching a maximum at t = 2.75 ± 0.25 sec after the start of the stimulus. [DA]o was elevated significantly, as compared with control, only after 0.75 sec of stimulation. In contrast, D2 agonism by quinpirole (□) was most prominent in the first 0.75 sec of stimulation by eliminating the initial fast rise phase. [DA]o increased gradually during the stimulus, reaching a maximum at t = 3.0 ± 0.25 sec after the start of the stimulus. Data are the mean ± SEM; times (s) are ± 0.25 sec; n = 7–26.
Fig. 5.
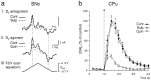
Typical voltammograms and traces of the effect of D2 receptor modulation on [DA]o evoked by a 100 Hz pseudo-one pulse stimulation in SNc and_CPu_. a, Typical voltammograms of maximum [DA]o obtained during stimulation (100 Hz, 10 pulses) in_SNc_ in control (Cont, solid line) and with (i) sulpiride (Sulp, 1 μ
m
) or (ii) quinpirole (Quin, 1 μ
m
; dashed traces). DA oxidation current was unaffected by the presence of sulpiride but was reduced significantly by quinpirole. Shown are the peak oxidation and reduction potentials of DA at +530 and −180 mV, respectively, versus Ag/AgCl (dotted lines). The voltammograms are scaled to illustrate relative concentrations. Electrode sensitivity to DA was 7.8–14.8 nA/μ
m
. Scale bars: 1 nA and 1 msec. iv, The applied FCV voltage waveform versus Ag/AgCl. b, Averaged traces of evoked [DA]o versus time during stimulation (100 Hz, 10 pulses) in CPu. Stimulation was at t = 1.0 sec (asterisk). In control (•), [DA]oreached maximum at 0.5 ± 0.25 sec after the start of the stimulus that followed a fast rise phase and then declined during stimulation. Control values are derived from controls for both sulpiride and quinpirole experiments. D2 antagonism by sulpiride (▴) had no significant effect on [DA]o or time to peak maximum. In contrast, D2 agonism by quinpirole (□) significantly reduced DA release, as compared with controls. Time to peak maximum remained unaffected. Data are the mean ± SEM; times are ± 0.25 sec; n = 10–22.
Fig. 1.
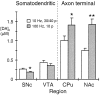
Evoked dopamine release in somatodendritic and axon terminal regions with varying pulse protocol. The graph illustrates the mean maximum evoked [DA]o in somatodendritic regions, SNc and VTA, and axon terminal regions, CPu and NAc, with sustained stimulus trains (10 Hz, 30/40 pulses (p), unfilled bars) versus a pseudo-one pulse stimulus (100 Hz, 10 pulses; filled bars). Evoked [DA]o was consistently greater in terminal than in somatodendritic regions. Evoked [DA]o was significantly less with the pseudo-one pulse stimulus than with the sustained lower frequency stimulus in SNc (*p < 0.05) and tended toward a similar decrease in VTA. In contrast, evoked [DA]o was significantly greater with the pseudo-one pulse stimulus than with the sustained lower frequency stimulus in both CPu(*p < 0.05) and NAc(**p < 0.01); n = 14–38.
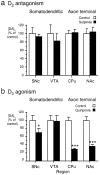
Fig. 2.
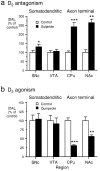
Summary of the effect of D2 receptor modulation on [DA]o evoked by a 10 Hz sustained stimulation in somatodendritic and axon terminal regions. Shown are the effects of (a) D2 antagonism and (b) D2 agonism on evoked [DA]o(10 Hz, 30/40 pulses) in somatodendritic regions and terminal fields of the A9 and A10 populations. a, Sulpiride (1 μ
m
) significantly increased [DA]o, as compared with controls, in SNc (*p < 0.05), but not in VTA_ (p > 0.05). Sulpiride significantly increased [DA]o, as compared with controls, in both dorsal CPu(***p < 0.001) and NAc(**p < 0.01). The degree of modulation of evoked [DA]o in CPu by sulpiride was significantly greater (p < 0.001) than in_SNc; n = 3–16. b, Quinpirole (1 μ
m
) had no significant effect, as compared with controls, in SNc (p > 0.05) or VTA (p > 0.05). In contrast, quinpirole significantly reduced [DA]o, as compared with controls, in both dorsal CPu(***p < 0.001) and NAc(**p < 0.01); n ranges from 10 to 25. Data are the mean percentage of control ± SEM.
Fig. 4.
Summary of the effect of D2 receptor modulation on [DA]o evoked by a 100 Hz pseudo-one pulse stimulation in somatodendritic and axon terminal regions. Shown are the effects of (a) D2 antagonism and (b) D2 agonism on evoked [DA]o(100 Hz, 10 pulses) in somatodendritic regions and terminal fields of the A9 and A10 populations. a, Sulpiride (1 μ
m
) had no significant effect on evoked [DA]o (100 Hz, 10 pulses) in either of the somatodendritic regions SNc or VTA or the terminal fields CPu or NAc of the A9 or A10 system (p > 0.05); _n_ranges from 5 to 10. b, Quinpirole (1 μ
m
) significantly reduced evoked [DA]o, as compared with controls, in SNc (*p < 0.05) but had no effect in VTA_ (_p_> 0.05). In contrast, quinpirole significantly reduced [DA]o, as compared with controls, in both dorsal_CPu (***p < 0.001) and_NAc_ (***p < 0.001);n = 8–23. Data are the mean percentage of control ± SEM.
Similar articles
- Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum.
Cragg S, Rice ME, Greenfield SA. Cragg S, et al. J Neurophysiol. 1997 Feb;77(2):863-73. doi: 10.1152/jn.1997.77.2.863. J Neurophysiol. 1997. PMID: 9065855 - Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro.
Rice ME, Cragg SJ, Greenfield SA. Rice ME, et al. J Neurophysiol. 1997 Feb;77(2):853-62. doi: 10.1152/jn.1997.77.2.853. J Neurophysiol. 1997. PMID: 9065854 - Comparison of serotonin and dopamine release in substantia nigra and ventral tegmental area: region and species differences.
Cragg SJ, Hawkey CR, Greenfield SA. Cragg SJ, et al. J Neurochem. 1997 Dec;69(6):2378-86. doi: 10.1046/j.1471-4159.1997.69062378.x. J Neurochem. 1997. PMID: 9375669 - Somatodendritic dopamine release: recent mechanistic insights.
Rice ME, Patel JC. Rice ME, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Jul 5;370(1672):20140185. doi: 10.1098/rstb.2014.0185. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26009764 Free PMC article. Review. - Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain.
Bustos G, Abarca J, Campusano J, Bustos V, Noriega V, Aliaga E. Bustos G, et al. Brain Res Brain Res Rev. 2004 Dec;47(1-3):126-44. doi: 10.1016/j.brainresrev.2004.05.002. Brain Res Brain Res Rev. 2004. PMID: 15572168 Review.
Cited by
- Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study.
Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Wu Q, et al. J Neurosci. 2002 Jul 15;22(14):6272-81. doi: 10.1523/JNEUROSCI.22-14-06272.2002. J Neurosci. 2002. PMID: 12122086 Free PMC article. - Local modulation by presynaptic receptors controls neuronal communication and behaviour.
Lovinger DM, Mateo Y, Johnson KA, Engi SA, Antonazzo M, Cheer JF. Lovinger DM, et al. Nat Rev Neurosci. 2022 Apr;23(4):191-203. doi: 10.1038/s41583-022-00561-0. Epub 2022 Feb 28. Nat Rev Neurosci. 2022. PMID: 35228740 Free PMC article. Review. - Biophysical Properties of Somatic and Axonal Voltage-Gated Sodium Channels in Midbrain Dopaminergic Neurons.
Yang J, Xiao Y, Li L, He Q, Li M, Shu Y. Yang J, et al. Front Cell Neurosci. 2019 Jul 10;13:317. doi: 10.3389/fncel.2019.00317. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31354436 Free PMC article. - Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function.
Martel P, Leo D, Fulton S, Bérard M, Trudeau LE. Martel P, et al. PLoS One. 2011;6(5):e20402. doi: 10.1371/journal.pone.0020402. Epub 2011 May 27. PLoS One. 2011. PMID: 21647367 Free PMC article. - Sustained N-methyl-d-aspartate receptor hypofunction remodels the dopamine system and impairs phasic signaling.
Ferris MJ, Milenkovic M, Liu S, Mielnik CA, Beerepoot P, John CE, España RA, Sotnikova TD, Gainetdinov RR, Borgland SL, Jones SR, Ramsey AJ. Ferris MJ, et al. Eur J Neurosci. 2014 Jul;40(1):2255-63. doi: 10.1111/ejn.12594. Epub 2014 Apr 23. Eur J Neurosci. 2014. PMID: 24754704 Free PMC article.
References
- Aceves J, Floran B, Martinez-Fong D, Benitez J, Sierra A, Flores G. Activation of D1 receptors stimulates accumulation of gamma-aminobutyric acid slices of the pars reticulata of 6-hydroxydopamine-lesioned rats. Neurosci Lett. 1992;145:40–42. - PubMed
- Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single-cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:1–7. - PubMed
- Björklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. - PubMed
- Blanchard V, Raisman-Vozari R, Vyas S, Michel PP, Javoy-Agid F, Uhl G, Agid Y. Differential expression of tyrosine hydroxylase and membrane dopamine transporter genes in subpopulations of dopaminergic neurons of the rat mesencephalon. Mol Brain Res. 1994;22:29–40. - PubMed
- Bouthenet M-L, Martres MP, Sales N, Schwartz JC. A detailed mapping of dopamine D2 receptors in rat central nervous system by autoradiography with [125I]-iodosulpiride. Neuroscience. 1987;20:117–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous