Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain - PubMed (original) (raw)
Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain
H G Kuhn et al. J Neurosci. 1997.
Abstract
Neurons and glia are generated throughout adulthood from proliferating cells in two regions of the rat brain, the subventricular zone (SVZ) and the hippocampus. This study shows that exogenous basic fibroblast growth factor (FGF-2) and epidermal growth factor (EGF) have differential and site-specific effects on progenitor cells in vivo. Both growth factors expanded the SVZ progenitor population after 2 weeks of intracerebroventricular administration, but only FGF-2 induced an increase in the number of newborn cells, most prominently neurons, in the olfactory bulb, the normal destination for neuronal progenitors migrating from the SVZ. EGF, on the other hand, reduced the total number of newborn neurons reaching the olfactory bulb and substantially enhanced the generation of astrocytes in the olfactory bulb. Moreover, EGF increased the number of newborn cells in the striatum either by migration of SVZ cells or by stimulation of local progenitor cells. No evidence of neuronal differentiation of newborn striatal cells was found by three-dimensional confocal analysis, although many of these newborn cells were associated closely with striatal neurons. The proliferation of hippocampal progenitors was not affected by either growth factor. However, EGF increased the number of newborn glia and reduced the number of newborn neurons, similar to the effects seen in the olfactory bulb. These findings may be useful for elucidating the in vivo role of growth factors in neurogenesis in the adult CNS and may aid development of neuronal replacement strategies after brain damage.
Figures
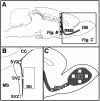
Fig. 1.
Analysis of the subventricular zone (SVZ) and olfactory bulb. A, Sagittal view of the rat brain illustrating the anatomical sites of progenitor proliferation in the SVZ, migration along the rostral migratory stream (RMS), and differentiation in the olfactory bulb (OB). Hatched bar_indicates position of coronal view in B.B, Coronal plane of the lateral ventricle with the corpus callosum (CC), medial septum (MS), and striatum (Str). Three areas in the_SVZ (ventral, lateral, and dorsal_squares_, 50 × 50 μm) and one area in the striatum (large rectangle, 300 × 600 μm) were analyzed for BrdU-positive cells on each section. C, Parasagittal plane of frontal cortex and olfactory bulb. Two areas of the RMS (small squares, 50 × 50 μm) and four areas of the OB granule cell layer (large squares, 100 × 100 μm) were analyzed for BrdU-positive cells and colabeling with NeuN or S100β.
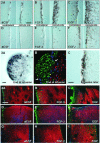
Fig. 2.
BrdU-positive cells in the SVZ at the end of and 4 weeks after intracerebroventricular infusion of aCSF (A, D), FGF-2 (B,E), and EGF (C, F). Note the large expansion of the SVZ and the density of newborn cells in the striatum after FGF-2 administration (B), which are even more dramatic after EGF administration (C). Proliferation was more pronounced on the side of the cannula, as compared with the contralateral side. Four weeks after growth factor withdrawal, a high density of BrdU-positive cells was still present in the SVZ of EGF-treated animals (F). Scale bar in A, 50 μm.
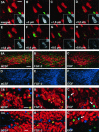
Fig. 3.
“Polyp-like” hyperplasia in the SVZ of EGF-treated animals at the end of treatment (2 weeks).A, High density of BrdU-positive cells at the convex pole of a hyperplasia, which protrudes into the CSF-filled ventricle.B, BrdU-positive cells are immunonegative for neuronal (NeuN, red) and astrocytic markers (S100β,blue). The ependymal layer (S100β,blue) is discontinuous (arrows) in areas of growth. C, Density of BrdU-labeled cells is still increased; however, the hyperplastic changes completely regress 4 weeks after EGF withdrawal. Scale bars in A, C, 25 μm.
Similar articles
- EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain.
Türeyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ. Türeyen K, et al. Neurosurgery. 2005 Dec;57(6):1254-63; discussion 1254-63. doi: 10.1227/01.neu.0000186040.96929.8a. Neurosurgery. 2005. PMID: 16331174 - Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain.
Gritti A, Frölichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Gritti A, et al. J Neurosci. 1999 May 1;19(9):3287-97. doi: 10.1523/JNEUROSCI.19-09-03287.1999. J Neurosci. 1999. PMID: 10212288 Free PMC article. - Induction of striatal neurogenesis and generation of region-specific functional mature neurons after ischemia by growth factors. Laboratory investigation.
Yoshikawa G, Momiyama T, Oya S, Takai K, Tanaka J, Higashiyama S, Saito N, Kirino T, Kawahara N. Yoshikawa G, et al. J Neurosurg. 2010 Oct;113(4):835-50. doi: 10.3171/2010.2.JNS09989. J Neurosurg. 2010. PMID: 20345217 - Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia.
Vicario-Abejón C, Yusta-Boyo MJ, Fernández-Moreno C, de Pablo F. Vicario-Abejón C, et al. J Neurosci. 2003 Feb 1;23(3):895-906. doi: 10.1523/JNEUROSCI.23-03-00895.2003. J Neurosci. 2003. PMID: 12574418 Free PMC article.
Cited by
- Excess HB-EGF, which promotes VEGF signaling, leads to hydrocephalus.
Shim JW, Sandlund J, Hameed MQ, Blazer-Yost B, Zhou FC, Klagsbrun M, Madsen JR. Shim JW, et al. Sci Rep. 2016 May 31;6:26794. doi: 10.1038/srep26794. Sci Rep. 2016. PMID: 27243144 Free PMC article. - Adult neurogenesis in mammals: an identity crisis.
Rakic P. Rakic P. J Neurosci. 2002 Feb 1;22(3):614-8. doi: 10.1523/JNEUROSCI.22-03-00614.2002. J Neurosci. 2002. PMID: 11826088 Free PMC article. Review. No abstract available. - Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model.
Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Takagi Y, et al. J Clin Invest. 2005 Jan;115(1):102-9. doi: 10.1172/JCI21137. J Clin Invest. 2005. PMID: 15630449 Free PMC article. - Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration.
Kim Y, Comte I, Szabo G, Hockberger P, Szele FG. Kim Y, et al. PLoS One. 2009 Dec 2;4(12):e8122. doi: 10.1371/journal.pone.0008122. PLoS One. 2009. PMID: 19956583 Free PMC article. - Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway.
Parent JM, Valentin VV, Lowenstein DH. Parent JM, et al. J Neurosci. 2002 Apr 15;22(8):3174-88. doi: 10.1523/JNEUROSCI.22-08-03174.2002. J Neurosci. 2002. PMID: 11943819 Free PMC article.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. - PubMed
- Berger F, Laine M, Hoffmann D, Verna JM, Charffanet M, Chauvin C, Rost N, Nissou MF, Benabid AL. The EGF receptor pathway in human cerebral tumors. Neurochirurgie. 1992;38:257–266. - PubMed
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. - PubMed
- Bonfanti L, Theodosis DT. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension, and in the olfactory bulb. Neuroscience. 1994;62:291–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical