Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo - PubMed (original) (raw)
Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo
B J Allen et al. J Neurosci. 1997.
Abstract
Dorsal root ganglia (DRG) neurons synthesize and transport substance P (SP) to the spinal cord where it is released in response to intense noxious somatosensory stimuli. We have shown previously that SP release in vivo causes a rapid and reversible internalization of SP receptors (SPRs) in dorsal horn neurons, which may provide a pharmacologically specific image of neurons activated by SP. Here, we report that noxious heat (43 degrees, 48 degrees, and 55 degrees C) and cold (10 degrees, 0 degrees, -10 degrees, and -20 degrees C) stimuli, but not innocuous warm (38 degrees C) and cold (20 degrees C) stimuli, applied to the hindpaw of anesthetized rats induce SPR internalization in spinal cord neurons that is graded with respect to the intensity of the thermal stimulus. Thus, with increasing stimulus intensities, both the total number of SPR+ lamina I neurons showing SPR internalization and the number of internalized SPR+ endosomes within each SPR immunoreactive neuron showed a significant increase. These data suggest that thermal stimuli induce a graded release of SP from primary afferent terminals and that agonist dependent receptor endocytosis provides evidence of a spatially and pharmacologically unique "neurochemical signature" after specific somatosensory stimuli.
Figures
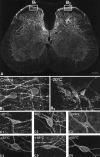
Fig. 1.
Confocal images of SPR immunoreactivity in the rat lumbar spinal cord. A, Confocal image of the rat spinal cord (L4 segment) 8 min after the plantar surface of the right hindpaw was stimulated for 30 sec with a 55°C thermode. The_boxes_ show the areas of the dorsal horn of the spinal cord that were sampled on the contralateral and stimulated sides. This medial aspect of the spinal cord is also the area that receives nociceptive inputs from the plantar surface of the hindpaw. In_A_ the SPR immunoreactivity appears white, whereas in B and C panels the SPR immunoreactivity appears gray (low levels) and_white_ (high levels). B1,B2, Confocal images, projected from 11 optical sections acquired at 0.7 μm intervals, of SPR immunoreactivity in the contralateral and ipsilateral sides, respectively, of the lumbar spinal cord after a −20°C stimulus to the right hindpaw. In the contralateral, unstimulated dorsal horn (B1), the SPR immunoreactivity is present on the plasma membrane, whereas on the stimulated side (B2), the SPR immunoreactivity is associated mainly with SPR+ endosomes.C1–C6, Confocal photomicrographs, projected from 22 optical sections acquired at 0.7 μm intervals, of SPR+ lamina I cell bodies 8 min after a single thermal stimulus was delivered to the hindpaw. In the contralateral control (C3) and in the ipsilateral spinal cord after the 32°C stimulus (C4), the SPR immunoreactivity is associated with the cell surface. After noxious thermal stimuli, the cell bodies experience a loss of immunoreactivity from the cell surface and an increase in the number of SPR+ endosomes in the neuronal cell body (C1, C2, C5,C6), suggesting that there is a graded release of SP from the primary afferents that is correlated with the intensity of the noxious thermal stimulation. Scale bars: A, 0.4 mm;B1–B2, 35 μm; C1–C6, 20 μm.
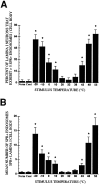
Fig. 2.
A, Mean proportion of SPR immunoreactive lamina I cell bodies exhibiting more than five SPR immunoreactive endosomes per SPR immunoreactive lamina I cell body as a function of stimulus intensity. B, Mean (±SEM) number of SPR+ endosomes per cell body in SPR immunoreactive lamina I neurons. In both cases the thermal stimulus group was compared with the 32°C contralateral control group, and the neurons sampled were present in the medial aspect of the rat L4 spinal segment. *p< 0.05.
Similar articles
- Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury.
Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Allen BJ, et al. J Neurophysiol. 1999 Mar;81(3):1379-90. doi: 10.1152/jn.1999.81.3.1379. J Neurophysiol. 1999. PMID: 10085363 - Stimulus specificity of peripherally evoked substance P release from the rabbit dorsal horn in situ.
Kuraishi Y, Hirota N, Sato Y, Hanashima N, Takagi H, Satoh M. Kuraishi Y, et al. Neuroscience. 1989;30(1):241-50. doi: 10.1016/0306-4522(89)90369-2. Neuroscience. 1989. PMID: 2473412 - Tooth extraction-induced internalization of the substance P receptor in trigeminal nucleus and spinal cord neurons: imaging the neurochemistry of dental pain.
Sabino MA, Honore P, Rogers SD, Mach DB, Luger NM, Mantyh PW. Sabino MA, et al. Pain. 2002 Jan;95(1-2):175-86. doi: 10.1016/s0304-3959(01)00397-9. Pain. 2002. PMID: 11790480 - Substance P in nociceptive sensory neurons.
Jessell TM. Jessell TM. Ciba Found Symp. 1982;(91):225-48. doi: 10.1002/9780470720738.ch13. Ciba Found Symp. 1982. PMID: 6183072 Review. - [Transmission and modulation of nociceptive information in the spinal dorsal horn].
Satoh M. Satoh M. Nihon Yakurigaku Zasshi. 1993 May;101(5):289-98. doi: 10.1254/fpj.101.5_289. Nihon Yakurigaku Zasshi. 1993. PMID: 8392482 Review. Japanese.
Cited by
- Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis.
Yu SJ, Grider JR, Gulick MA, Xia CM, Shen S, Qiao LY. Yu SJ, et al. Exp Neurol. 2012 Dec;238(2):209-17. doi: 10.1016/j.expneurol.2012.08.007. Epub 2012 Aug 19. Exp Neurol. 2012. PMID: 22921460 Free PMC article. - Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila.
Im SH, Takle K, Jo J, Babcock DT, Ma Z, Xiang Y, Galko MJ. Im SH, et al. Elife. 2015 Nov 17;4:e10735. doi: 10.7554/eLife.10735. Elife. 2015. PMID: 26575288 Free PMC article. - Tachykinin NK₁ receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation.
Tumati S, Largent-Milnes TM, Keresztes AI, Yamamoto T, Vanderah TW, Roeske WR, Hruby VJ, Varga EV. Tumati S, et al. Eur J Pharmacol. 2012 Jun 5;684(1-3):64-70. doi: 10.1016/j.ejphar.2012.03.025. Eur J Pharmacol. 2012. PMID: 22724132 Free PMC article. - Peptidases prevent mu-opioid receptor internalization in dorsal horn neurons by endogenously released opioids.
Song B, Marvizón JC. Song B, et al. J Neurosci. 2003 Mar 1;23(5):1847-58. doi: 10.1523/JNEUROSCI.23-05-01847.2003. J Neurosci. 2003. PMID: 12629189 Free PMC article. - Spinal N-methyl-D-aspartate receptors and nociception-evoked release of primary afferent substance P.
Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. Nazarian A, et al. Neuroscience. 2008 Mar 3;152(1):119-27. doi: 10.1016/j.neuroscience.2007.11.037. Neuroscience. 2008. PMID: 18222611 Free PMC article.
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. - PubMed
- Aimone LD, Yaksh TL. Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides. 1989;10:1127–1131. - PubMed
- Benya RV, Fathi Z, Kusui T, Pradhan T, Battey JF, Jensen RT. Gastrin-releasing peptide receptor-induced internalization, down-regulation, desensitization, and growth: possible role for cyclic AMP. Mol Pharmacol. 1994;46:235–245. - PubMed
- Boehmer CG, Norman J, Catton M, Fine LG, Mantyh PW. High levels of mRNA coding for substance P, somatostatin and α-tubulin are expressed by rat and rabbit dorsal root ganglia neurons. Peptides. 1989;10:1179–1194. - PubMed
- Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources