A genetic animal model of human neocortical heterotopia associated with seizures - PubMed (original) (raw)
. 1997 Aug 15;17(16):6236-42.
doi: 10.1523/JNEUROSCI.17-16-06236.1997.
F Schottler, J L Collins, G Lanzino, D Couture, A Rao, K Hiramatsu, Y Goto, S C Hong, H Caner, H Yamamoto, Z F Chen, E Bertram, S Berr, R Omary, H Scrable, T Jackson, J Goble, L Eisenman
Affiliations
- PMID: 9236234
- PMCID: PMC6568362
- DOI: 10.1523/JNEUROSCI.17-16-06236.1997
A genetic animal model of human neocortical heterotopia associated with seizures
K S Lee et al. J Neurosci. 1997.
Abstract
Malformations of the human neocortex are commonly associated with developmental delays, mental retardation, and epilepsy. This study describes a novel neurologically mutant rat exhibiting a forebrain anomaly resembling the human neuronal migration disorder of double cortex. This mutant displays a telencephalic internal structural heterotopia (tish) that is inherited in an autosomal recessive manner. The bilateral heterotopia is prominent below the frontal and parietal neocortices but is rarely observed in temporal neocortex. Neurons in the heterotopia exhibit neocortical-like morphologies and send typical projections to subcortical sites; however, characteristic lamination and radial orientation are disturbed in the heterotopia. The period of neurogenesis during which cells in the heterotopia are generated is the same as in the normotopic neocortex; however, the cells in the heterotopia exhibit a "rim-to-core" neurogenetic pattern rather than the characteristic "inside-out" pattern observed in normotopic neocortex. Similar to the human syndrome of double cortex, some of the animals with the tish phenotype exhibit spontaneous recurrent electrographic and behavioral seizures. The tish rat is a unique neurological mutant that shares several features with a human cortical malformation associated with epilepsy. On the basis of its regional connectivity, histological composition, and period of neurogenesis, the heterotopic region in the tish rat is neocortical in nature. This neurological mutant represents a novel model system for investigating mechanisms of aberrant neocortical development and is likely to provide insights into the cellular and molecular events contributing to seizure development in dysplastic neocortex.
Figures
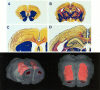
Fig. 1.
Top. Coronal sections of a_tish_ brain stained for AChE. Low-magnification photomicrographs (A, B) show the heterotopia to be a large bilateral structure intercalated between the neocortex and underlying white matter. A thin layer of white matter separates the heterotopia from the overlying neocortex, and a thicker layer of white matter separates it from lower structures. In_A_ and C, the heterotopia is located above the darkly stained striatum and below the neocortex. In the more caudal sections (B, D), the heterotopia is located between the hippocampus (or lateral ventricle) and the neocortex. The heterotopia has a multinodular appearance attributable to the passage of one or more large fiber tracts through the structure.
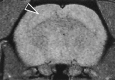
Fig. 3.
MR image of the tish brain. In this proton density image, the heterotopia can be observed bilaterally as an area isodense to the overlying normotopic cortex (arrowhead indicates structure on the right side of the brain). MRI was performed at the Small Aperture Magnetic Imaging and Spectroscopy Center at the University of Virginia using a 4.7 Tesla 40 cm bore 200/400 MR imager.
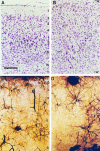
Fig. 4.
Histological appearance of the normotopic neocortex and heterotopic region of a tish animal. Photomicrographs of Nissl-stained sections are shown of the normotopic neocortex (A) and heterotopia (B). Cellular somata in the normotopic neocortex exhibit a laminar organization, whereas cells in the heterotopia do not exhibit strict lamination. Golgi-stained sections of the normotopic neocortex (C) and heterotopic region (D) demonstrate the presence of similar cell types in the two structures. In the normotopic neocortex, the apical dendrites of pyramidal neurons are oriented radially, and their somata are located in appropriate laminae. In contrast, the neurons in the heterotopia do not exhibit a strict laminar pattern and their apical dendrites are not oriented uniformly. Numbers indicate layers of the neocortex. W, White matter;T, heterotopia. Scale bars: A, B, 200 μm; C, D, 170 μm.
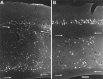
Fig. 5.
Neurogenetic patterns in the forebrain of the tish rat. Photomicrographs are shown of coronal sections of tish brains in which immunostaining was performed on animals labeled with BrDU on E15 (A) or E18 (B); these animals survived to P33 and P30, respectively. Darkly stained (BrDU-positive) cells are found primarily in the deep aspect of the normotopic neocortex in the E15-injected animal (A) and in the superficial aspect of the normotopic neocortex in the E18-injected animal (B). Labeled cells are located primarily in the rim of the heterotopia in the E15-injected animal (A) and in the core of the heterotopia in the E18-injected animal (B). _Arrows_indicate the white matter surrounding the heterotopic region. Scale bar, 200 μm.
Fig. 6.
Subcortical connectivity of the_tish_ forebrain. Fluorescence micrographs show retrogradely labeled projection neurons in both the normotopic neocortex and neighboring heterotopia after injection of Fluorogold into either the thalamus (A) or spinal cord (B). After an injection into the VPL of the thalamus (A), the somata of labeled neurons in the normotopic neocortex are located primarily in layer VI, and their apical dendrites are radially oriented. Labeled cells in the heterotopic region are similar in size and appearance to those in the normotopic neocortex but are more widely dispersed and tend to be concentrated along the rim area of the heterotopia. Injection of Fluorogold into the cervical spinal cord labels large pyramidal cells in layer V of the normotopic neocortex; the apical dendrites of these cells exhibit a typical radial orientation. The heterotopia also contains retrogradely labeled pyramidal neurons; however, these cells are not laminated and their apical dendrites are not consistently oriented toward the surface of the brain. _Arrows_indicate the white matter surrounding the heterotopia. Scale bar, 200 μm.
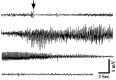
Fig. 7.
Electroencephalographic recordings of a seizure in a tish rat. The four lines represent a continuous EEG recording from a single electrode positioned in the normotopic neocortex. Seizure activity can be observed as changes in the frequency and amplitude of the EEG; this event lasted ∼63 sec. The animal exhibited convulsive behavioral seizures during the electrographic seizure event (arrow indicates seizure onset).
Similar articles
- Distribution and initiation of seizure activity in a rat brain with subcortical band heterotopia.
Chen ZF, Schottler F, Bertram E, Gall CM, Anzivino MJ, Lee KS. Chen ZF, et al. Epilepsia. 2000 May;41(5):493-501. doi: 10.1111/j.1528-1157.2000.tb00201.x. Epilepsia. 2000. PMID: 10802753 - Heterotopic neurogenesis in a rat with cortical heterotopia.
Lee KS, Collins JL, Anzivino MJ, Frankel EA, Schottler F. Lee KS, et al. J Neurosci. 1998 Nov 15;18(22):9365-75. doi: 10.1523/JNEUROSCI.18-22-09365.1998. J Neurosci. 1998. PMID: 9801375 Free PMC article. - Normotopic and heterotopic cortical representations of mystacial vibrissae in rats with subcortical band heterotopia.
Schottler F, Fabiato H, Leland JM, Chang LY, Lotfi P, Getachew F, Lee KS. Schottler F, et al. Neuroscience. 2001;108(2):217-35. doi: 10.1016/s0306-4522(01)00395-5. Neuroscience. 2001. PMID: 11734356 - In utero irradiation of rats as a model of human cerebrocortical dysgenesis: a review.
Roper SN. Roper SN. Epilepsy Res. 1998 Sep;32(1-2):63-74. doi: 10.1016/s0920-1211(98)00040-0. Epilepsy Res. 1998. PMID: 9761309 Review. - [Cortical heterotopias: animal models and human disease].
Chevassus-au-Louis N, Robain O. Chevassus-au-Louis N, et al. Rev Neurol (Paris). 1999 Jan;155(1):51-8. Rev Neurol (Paris). 1999. PMID: 10093850 Review. French.
Cited by
- Neocortical and cerebellar malformations affect flurothyl-induced seizures in female C57BL/6J mice.
Keever KM, Li Y, Womble PD, Sullens DG, Otazu GH, Lugo JN, Ramos RL. Keever KM, et al. Front Neurosci. 2023 Nov 2;17:1271744. doi: 10.3389/fnins.2023.1271744. eCollection 2023. Front Neurosci. 2023. PMID: 38027492 Free PMC article. - Novel role of the synaptic scaffold protein Dlgap4 in ventricular surface integrity and neuronal migration during cortical development.
Romero DM, Poirier K, Belvindrah R, Moutkine I, Houllier A, LeMoing AG, Petit F, Boland A, Collins SC, Soiza-Reilly M, Yalcin B, Chelly J, Deleuze JF, Bahi-Buisson N, Francis F. Romero DM, et al. Nat Commun. 2022 May 18;13(1):2746. doi: 10.1038/s41467-022-30443-z. Nat Commun. 2022. PMID: 35585091 Free PMC article. - Intravital Imaging of Neocortical Heterotopia Reveals Aberrant Axonal Pathfinding and Myelination around Ectopic Neurons.
Li AM, Hill RA, Grutzendler J. Li AM, et al. Cereb Cortex. 2021 Jul 29;31(9):4340-4356. doi: 10.1093/cercor/bhab090. Cereb Cortex. 2021. PMID: 33877363 Free PMC article. - Targeted Neuronal Injury for the Non-Invasive Disconnection of Brain Circuitry.
Wang W, Zhang Y, Anzivino MJ, Bertram EH, Woznak J, Klibanov A, Dumont E, Wintermark M, Lee KS. Wang W, et al. J Vis Exp. 2020 Sep 27;(163):10.3791/61271. doi: 10.3791/61271. J Vis Exp. 2020. PMID: 33044450 Free PMC article. - A deletion in Eml1 leads to bilateral subcortical heterotopia in the tish rat.
Grosenbaugh DK, Joshi S, Fitzgerald MP, Lee KS, Wagley PK, Koeppel AF, Turner SD, McConnell MJ, Goodkin HP. Grosenbaugh DK, et al. Neurobiol Dis. 2020 Jul;140:104836. doi: 10.1016/j.nbd.2020.104836. Epub 2020 Mar 13. Neurobiol Dis. 2020. PMID: 32179177 Free PMC article.
References
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
- Barkovich AJ, Jackson DE, Boyer RS. Band heterotopias: a newly recognized neuronal migration anomaly. Radiology. 1989;171:455–458. - PubMed
- Bertram EH, Cornett J. The ontogeny of seizures in a rat model of limbic epilepsy: evidence for a kindling process in the development of chronic spontaneous seizures. Brain Res. 1993;625:295–300. - PubMed
- Bertram EH, Cornett J. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994;661:157–162. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical