Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators - PubMed (original) (raw)
Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators
K Hyrc et al. J Neurosci. 1997.
Abstract
Cytosolic calcium ([Ca2+]i) is an important mediator of neuronal signal transduction, participating in diverse biochemical reactions that elicit changes in synaptic efficacy, metabolic rate, and gene transcription. Excessive [Ca2+]i also has been implicated as a cause of acute neuronal injury, although measurement of [Ca2+]i in living neurons by fluorescent calcium indicators has not consistently demonstrated a correlation between [Ca2+]i and the likelihood of neuronal death after a variety of potentially lethal insults. Using fluorescence videomicroscopy and microinjected calcium indicators, we measured [Ca2+]i in cultured cortical neurons during intense activation with either NMDA (300 microM) or AMPA (450 microM). At these concentrations NMDA killed >80% of the cultured neurons by the next day, whereas neuronal death from AMPA was <20%. Using the conventional calcium indicator, fura-2/AM, we estimated [Ca2+]i elevations to be approximately 300-400 nM during exposure to either glutamate agonist. In contrast, indicators with lower affinity for calcium, benzothiazole coumarin (BTC), and fura-2/dextran reported [Ca2+]i levels >5 microM during lethal NMDA exposure, but [Ca2+]i levels were <1.5 microM during nonlethal activation of AMPA receptors or voltage-gated calcium channels. Fura-2 reported [Ca2+]i responses during brief exposure to glutamate, NMDA, AMPA, kainate, and elevated extracellular K+ between 0.5 and 1 microM. With the use of BTC, only NMDA and glutamate exposures resulted in micromolar [Ca2+]i levels. Neurotoxic glutamate receptor activation is associated with sustained, micromolar [Ca2+]i elevation. The widely used calcium indicator fura-2 selectively underestimates [Ca2+]i, depending on the route of entry, even at levels that appear to be within its range of detection.
Figures
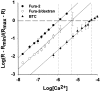
Fig. 1.
Ca2+ dissociation constants for fura-2, fura-2/dextran (MW 3000), and BTC. Spectrofluorimetric calibration curves were obtained for each indicator at 5.0 μ
m
in buffered solutions over an appropriate range of calcium concentrations. The data shown are averages of three calibration experiments performed for each indicator (mean ± SD). To facilitate comparisons, the _x_-intercept and corresponding log[Ca2+] for each indicator are shown as dashed and dotted lines, respectively. Calculated _K_D values are as follows: fura-2 (•), 0.24 ± 0.04 μ
m
; fura-2/dextran (○), 0.94 ± 0.25 μ
m
; BTC (▴), 15.2 ± 0.12 μ
m
.
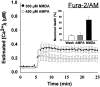
Fig. 2.
[Ca2+]i levels measured using fura-2/AM do not differ during nontoxic AMPA and toxic NMDA exposures. Cultured cortical neurons were loaded by incubation with 6 μ
m
fura-2/AM, and neuronal [Ca2+]i was determined by fluorescence ratio imaging as described (see Materials and Methods) in neurons exposed to 300 μ
m
NMDA (with 10 μ
m
glycine) or 450 μ
m
AMPA (with 10 μ
m
MK-801 to prevent NMDA receptor activation). Values represent mean (± SE) [Ca2+]i derived from 15–20 bath-loaded neurons in each of four to six dishes.Inset, Cultures were exposed for 20 min either to 300 μ
m
NMDA (with 10 μ
m
glycine) or to 450 μ
m
AMPA (with 10 μ
m
MK-801 to prevent NMDA receptor activation). After treatment, cells were washed with HBBSS with 10 μ
m
MK-801 and returned to the incubator. Cell viability was assessed after 20–28 hr by trypan blue exclusion. Values represent mean (± SD) percentage of nonviable neurons counted in 5–10 random fields in 10–15 dishes.
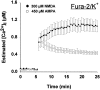
Fig. 3.
[Ca2+]i measured by microinjected fura-2. Single neurons were filled with 500 μ
m
fura-2 by transient patch micropipette application and exposed to AMPA or NMDA, as in Figure 2. Values represent mean ± SE [Ca2+]i from four to six neurons.
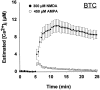
Fig. 4.
[Ca2+]i measured by microinjected BTC. Single neurons were filled with the low-affinity calcium indicator BTC (500 μ
m
;_K_D = 15 μ
m
) and exposed to AMPA or NMDA, as in Figure 2. Values represent mean ± SE [Ca2+]i from four to six neurons. BTC reveals large differences between NMDA- and AMPA-induced [Ca2+]i elevation.
Fig. 5.
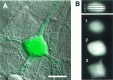
Cellular distribution of BTC in an individual dye-filled cortical neuron. Optical sections were taken at 0.5 μm intervals, using a laser scanning confocal microscope.A, A through-focus confocal image of BTC (green) overlayed on the Nomarski image (scale bar, 15 μm). B, The BTC fluorescence data (xz cross section) are shown in the upper right, with the indicated xy planes shown_below_ (scale for these images is 12 μm). BTC appears uniformly distributed throughout the neuronal cytoplasm.
Fig. 6.
[Ca2+]i levels measured with fura-2/dextran. Neurons were filled with fura-2 conjugated to 3000 MW dextran (500 μ
m
;_K_D = 0.94 μ
m
) and exposed to NMDA or AMPA as in Figure 2. Values represent mean ± SE [Ca2+]i from four to six neurons.
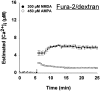
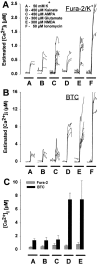
Fig. 7.
Fura-2 and BTC report markedly different [Ca2+]i levels even during sublethal exposure conditions. Neurons were exposed for 2 min to 50 m
m
KCl, 450 μ
m
kainate or AMPA, 300 μ
m
glutamate or NMDA, or 50 μ
m
ionomycin after microinjection with either fura-2 (A) or BTC (B). Each trace represents estimated [Ca2+]i from a single neuron. C, Mean ± SD [Ca2+]i elevation during the second minute of drug application in A or B. With the use of fura-2, measured [Ca2+]i increased to a similar level for each treatment except ionomycin (A, C). In contrast, measurements using BTC revealed a significant difference in [Ca2+]i among cells treated with KCl, kainate or AMPA, and cells exposed to NMDA or glutamate (B, C). In fura-2-filled neurons, ionomycin application elevated [Ca2+]i to a significantly greater extent than other drug treatments (A), suggesting that fura-2 was not saturated in these experiments.
Similar articles
- Rapid Ca2+ entry through Ca2+-permeable AMPA/Kainate channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production.
Carriedo SG, Yin HZ, Sensi SL, Weiss JH. Carriedo SG, et al. J Neurosci. 1998 Oct 1;18(19):7727-38. doi: 10.1523/JNEUROSCI.18-19-07727.1998. J Neurosci. 1998. PMID: 9742143 Free PMC article. - Brief calcium transients evoked by glutamate receptor agonists in rat dorsal horn neurons: fast kinetics and mechanisms.
Reichling DB, MacDermott AB. Reichling DB, et al. J Physiol. 1993 Sep;469:67-88. doi: 10.1113/jphysiol.1993.sp019805. J Physiol. 1993. PMID: 7505825 Free PMC article. - Evidence that the early loss of membrane protein kinase C is a necessary step in the excitatory amino acid-induced death of primary cortical neurons.
Durkin JP, Tremblay R, Chakravarthy B, Mealing G, Morley P, Small D, Song D. Durkin JP, et al. J Neurochem. 1997 Apr;68(4):1400-12. doi: 10.1046/j.1471-4159.1997.68041400.x. J Neurochem. 1997. PMID: 9084410 - Calcium dynamics in the central nervous system.
DeCoster MA. DeCoster MA. Adv Neuroimmunol. 1995;5(3):233-9. doi: 10.1016/0960-5428(95)00015-t. Adv Neuroimmunol. 1995. PMID: 8748068 Review. - Comprehensive review of indicators and techniques for optical mapping of intracellular calcium ions.
Sheng CQ, Wu SS, Cheng YK, Wu Y, Li YM. Sheng CQ, et al. Cereb Cortex. 2024 Aug 1;34(8):bhae346. doi: 10.1093/cercor/bhae346. Cereb Cortex. 2024. PMID: 39191664 Review.
Cited by
- Altered calcium signaling following traumatic brain injury.
Weber JT. Weber JT. Front Pharmacol. 2012 Apr 12;3:60. doi: 10.3389/fphar.2012.00060. eCollection 2012. Front Pharmacol. 2012. PMID: 22518104 Free PMC article. - Mitochondria and calcium: from cell signalling to cell death.
Duchen MR. Duchen MR. J Physiol. 2000 Nov 15;529 Pt 1(Pt 1):57-68. doi: 10.1111/j.1469-7793.2000.00057.x. J Physiol. 2000. PMID: 11080251 Free PMC article. Review. - Activity-dependent development of calcium regulation in growing motor axons.
Lnenicka GA, Arcaro KF, Calabro JM. Lnenicka GA, et al. J Neurosci. 1998 Jul 1;18(13):4966-72. doi: 10.1523/JNEUROSCI.18-13-04966.1998. J Neurosci. 1998. PMID: 9634562 Free PMC article. - Maintained LTP and Memory Are Lost by Zn2+ Influx into Dentate Granule Cells, but Not Ca2+ Influx.
Takeda A, Tamano H, Hisatsune M, Murakami T, Nakada H, Fujii H. Takeda A, et al. Mol Neurobiol. 2018 Feb;55(2):1498-1508. doi: 10.1007/s12035-017-0428-3. Epub 2017 Feb 7. Mol Neurobiol. 2018. PMID: 28176276 - Neuroprotective effects of vitamin D alone or in combination with lamotrigine against lithium-pilocarpine model of status epilepticus in rats.
Mahfoz AM, Abdel-Wahab AF, Afify MA, Shahzad N, Ibrahim IAA, ElSawy NA, Bamagous GA, Al Ghamdi SS. Mahfoz AM, et al. Naunyn Schmiedebergs Arch Pharmacol. 2017 Oct;390(10):977-985. doi: 10.1007/s00210-017-1400-5. Epub 2017 Jul 7. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28687854
References
- Augustine GJ, Neher E. Neuronal Ca2+ signaling takes the local route. Curr Opin Neurobiol. 1992;2:302–307. - PubMed
- Brocard JB, Rajdev S, Reynolds IJ. Glutamate-induced increases in intracellular free Mg2+ in cultured cortical neurons. Neuron. 1993;11:751–757. - PubMed
- Canzoniero LMT, Sensi SL, Choi DW. Recovery from NMDA-induced intracellular acidification is delayed and dependent on extracellular bicarbonate. Am J Physiol. 1996;39:C593–C599. - PubMed
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous