A role for TFIIH in controlling the activity of early RNA polymerase II elongation complexes - PubMed (original) (raw)
A role for TFIIH in controlling the activity of early RNA polymerase II elongation complexes
A Dvir et al. Proc Natl Acad Sci U S A. 1997.
Abstract
TFIIH is a multifunctional RNA polymerase II transcription factor that possesses DNA-dependent ATPase, DNA helicase, and protein kinase activities. Previous studies have established that TFIIH enters the preinitiation complex and fulfills a critical role in initiation by catalyzing ATP-dependent formation of the open complex prior to synthesis of the first phosphodiester bond of nascent transcripts. In this report, we present direct evidence that TFIIH also controls RNA polymerase II activity at a postinitiation stage of transcription, by preventing premature arrest by very early elongation complexes just prior to their transition to stably elongating complexes. Unexpectedly, we observe that TFIIH is capable of entering the transcription cycle not only during assembly of the preinitiation complex but also after initiation and synthesis of as many as four to six phosphodiester bonds. These findings shed new light on the role of TFIIH in initiation and promoter escape and reveal an unanticipated flexibility in the ability of TFIIH to interact with RNA polymerase II transcription intermediates prior to, during, and immediately after initiation.
Figures
Figure 1
Structures of the transcriptional start sites of the wild-type AdML and Ad(−9/−1) promoters. The DNA strands of the wild-type AdML promoter (Upper) are fully complementarty, whereas the Ad(−9/−1) promoter (Lower) contains a 9-bp mismatch just upstream of the in vivo transcriptional start site. The bottom (coding) strand of the Ad(−9/−1) promoter is identical to the wild-type AdML promoter sequence. ▾, Position of the in vivo transcriptional start site of the AdML promoter.
Figure 4
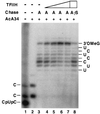
TFIIH can interact functionally with early RNA polymerase II elongation complexes to suppress promoter-proximal arrest. Transcription reactions were performed. Preinitiation complexes were assembled at the Ad(−9/−1) promoter in the absence of TFIIH. Transcription was initiated with 200 μM CpU, 5 μM ATP, 0.01 μM UTP, and 0.5 μM [α-32P]CTP). After a 15-min incubation at 28°C, elongation complexes containing short (5–7 nt) transcripts were separated from initiating nucleotides by AcA 34 gel filtration. Short transcripts were then chased into longer transcripts in the presence of 100 μM ATP (A) or 100 μM ATP[γS] (AγS) and various amounts of TFIIH. Transcripts were analyzed as described (12) by electrophoresis through a 25% acrylamide/3% N,N_′-methylenebisacrylamide/7.0 M urea gel. Lanes: 1, transcripts before AcA 34 gel filtration; 2, transcripts associated with isolated elongation complexes before the chase; 3–8, transcripts resulting from chase of isolated elongation complexes. The transcription reactions in lanes 4–8 contained ≈1.5 ng (lane 4), ≈6 ng (lane 5), ≈15 ng (lane 6), or ≈30 ng (lanes 7 and 8) of TFIIH (fraction 18 from TSK SP-5-PW fractionation shown in Fig. 5_D). TFIIH was added to reaction mixtures immediately before addition of chase nucleotides. The labels on either side of the figure indicate the nucleotides at the 3′ end of transcripts. 3′OMeG, 3′-_O_-MeG-terminated transcripts.
Figure 2
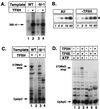
ATP-dependent suppression of promoter-proximal arrest requires TFIIH and TFIIE. (A) Run-off transcription from the AdML and Ad(−9/−1) promoters in the presence or absence of TFIIH. Transcription reactions were carried out. Transcription was initiated with 100 μM ATP, 100 μM GTP, 100 μM UTP, and 10 μM [α-32P]CTP and was carried out for 30 min at 28°C. Approximately 350-nt run-off transcripts were analyzed by electrophoresis through a 6% acrylamide/0.8% _N,N_′-methylenebisacrylamide/7.0 M urea gel. WT, wild type; −9/−1, Ad(−9/−1). (B) Kinetics of transcription initiation at the Ad(−9/−1) promoter. Dinucleotide-primed abortive initiation assays were performed as described (11) for the times indicated with 200 μM CpU and 0.5 μM [α-32P]CTP. Trinucleotide transcripts were analyzed as described (11) by electrophoresis through a 25% acrylamide/3% _N,N_′-methylenebisacrylamide/7.0 M urea gel. Lanes labeled All contained the complete set of general initiation factors. Lanes labeled −TFIIH did not contain TFIIH. (C) TFIIH dependence of transcription of the wild-type AdML and Ad(−9/−1) promoters. Transcription of the wild-type AdML and Ad(−9/−1) promoters were performed in the presence or absence of TFIIH as indicated. Transcripts were analyzed as described (12) by electrophoresis through a 25% acrylamide/3% _N,N_′-methylenebisacrylamide/7.0 M urea gel. WT, wild type; −9/−1, Ad(−9/−1); 3′OMeG stop, 3′-_O_-MeG-terminated transcripts. (D) ATP dependence of transcription of the Ad(−9/−1) promoter. Transcription reactions were performed in the presence or absence of either TFIIH or TFIIE and TFIIH. Transcription was initiated by addition of 200 μM CpA, 5 μM UTP, 0.5 μM [α-32P]CTP, and 100 μM 3′-_O_-MeGTP, with or without 5 μM ATP. Transcripts were analyzed as described (12) by electrophoresis through a 25% acrylamide/3% _N,N_′-methylenebisacrylamide/7.0 M urea gel. 3′OMeG stop, 3′-_O_-MeG-terminated transcripts.
Figure 3
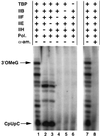
Transcription from the Ad(−9/−1) promoter depends on general initiation factors and is sensitive to inhibition by α-amanitin. Transcription was performed as described in Fig. 2_C_ in the presence of the indicated transcription factors, with or without α-amanitin at 1 μg/ml. Transcripts were analyzed as described (12) by electrophoresis through a 25% acrylamide/3% _N,N_′-methylenebisacrylamide/7.0 M urea gel. IIB, TFIIB; IIE, TFIIE; IIH, TFIIH; Pol., RNA polymerase II; α-am., α-amanitin; 3′OMeG; 3′-_O_-MeG-terminated transcripts.
Figure 5
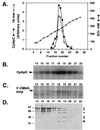
Cochromatography of TFIIH and ATP-dependent arrest suppressing activity. (A) Elution profile of TFIIH initiation activity and arrest suppressing activity during ion-exchange chromatography of purified TFIIH on TSK SP-5-PW. TSK SP-5-PW HPLC purification of TFIIH was performed as described (15). The amounts of dinucleotide-primed trinucleotide CpApC and 18-nt 3′-O_-MeG-terminated transcripts synthesized are expressed in arbitrary units determined by PhosphorImager analysis of polyacrylamide gels. (B) TFIIH initiation activity measured using the abortive initiation assay performed as described in Fig. 2_B. Trinucleotide transcripts were analyzed as described (11) by electrophoresis through a 25% acrylamide/3% _N,N_′-methylenebisacrylamide/7.0 M urea gel. (C) Pulse–chase assay for arrest suppressing activity. Transcription reactions were performed. Preinitiation complexes were assembled at the Ad(−9/−1) promoter in the absence of TFIIH. Transcription was initiated with 200 μM CpU, 5 μM ATP, 0.01 μM UTP, and 0.5 μM [α-32P]CTP. After a 15-min incubation at 28°C, short transcripts were chased into longer transcripts in the presence of the indicated TSK SP-5-PW fractions and 100 μM ATP, 100 μM UTP, 200 μM CTP, and 100 μM 3′-_O_-MeGTP. Transcripts were analyzed as described (12) by electrophoresis through a 25% acrylamide/3% _N,N_′-methylenebisacrylamide/7.0 M urea gel. (D) Polypeptide composition of TSK SP-5-PW fractions. TSK SP-5-PW fractions were analyzed by electrophoresis through an SDS/8% polyacrylamide gel and silver staining. •, TFIIH subunits; ⊙, two TFIIH subunits that were not well resolved in this gel (data not shown).
Similar articles
- A role for the TFIIH XPB DNA helicase in promoter escape by RNA polymerase II.
Moreland RJ, Tirode F, Yan Q, Conaway JW, Egly JM, Conaway RC. Moreland RJ, et al. J Biol Chem. 1999 Aug 6;274(32):22127-30. doi: 10.1074/jbc.274.32.22127. J Biol Chem. 1999. PMID: 10428772 - Dual roles for transcription factor IIF in promoter escape by RNA polymerase II.
Yan Q, Moreland RJ, Conaway JW, Conaway RC. Yan Q, et al. J Biol Chem. 1999 Dec 10;274(50):35668-75. doi: 10.1074/jbc.274.50.35668. J Biol Chem. 1999. PMID: 10585446 - A role for ATP and TFIIH in activation of the RNA polymerase II preinitiation complex prior to transcription initiation.
Dvir A, Garrett KP, Chalut C, Egly JM, Conaway JW, Conaway RC. Dvir A, et al. J Biol Chem. 1996 Mar 29;271(13):7245-8. doi: 10.1074/jbc.271.13.7245. J Biol Chem. 1996. PMID: 8631733 - The multiple roles of transcription/repair factor TFIIH.
Svejstrup JQ, Vichi P, Egly JM. Svejstrup JQ, et al. Trends Biochem Sci. 1996 Sep;21(9):346-50. Trends Biochem Sci. 1996. PMID: 8870499 Review. - Promoter escape by RNA polymerase II.
Dvir A. Dvir A. Biochim Biophys Acta. 2002 Sep 13;1577(2):208-223. doi: 10.1016/s0167-4781(02)00453-0. Biochim Biophys Acta. 2002. PMID: 12213653 Review.
Cited by
- P-TEFb kinase recruitment and function at heat shock loci.
Lis JT, Mason P, Peng J, Price DH, Werner J. Lis JT, et al. Genes Dev. 2000 Apr 1;14(7):792-803. Genes Dev. 2000. PMID: 10766736 Free PMC article. - Promoter-proximal stalling results from the inability to recruit transcription factor IIH to the transcription complex and is a regulated event.
Kumar KP, Akoulitchev S, Reinberg D. Kumar KP, et al. Proc Natl Acad Sci U S A. 1998 Aug 18;95(17):9767-72. doi: 10.1073/pnas.95.17.9767. Proc Natl Acad Sci U S A. 1998. PMID: 9707550 Free PMC article. - The carboxy terminus of the small subunit of TFIIE regulates the transition from transcription initiation to elongation by RNA polymerase II.
Watanabe T, Hayashi K, Tanaka A, Furumoto T, Hanaoka F, Ohkuma Y. Watanabe T, et al. Mol Cell Biol. 2003 Apr;23(8):2914-26. doi: 10.1128/MCB.23.8.2914-2926.2003. Mol Cell Biol. 2003. PMID: 12665589 Free PMC article. - RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells.
Jenkins HL, Spencer CA. Jenkins HL, et al. J Virol. 2001 Oct;75(20):9872-84. doi: 10.1128/JVI.75.20.9872-9884.2001. J Virol. 2001. PMID: 11559820 Free PMC article. - p53-Dependent p21 mRNA elongation is impaired when DNA replication is stalled.
Mattia M, Gottifredi V, McKinney K, Prives C. Mattia M, et al. Mol Cell Biol. 2007 Feb;27(4):1309-20. doi: 10.1128/MCB.01520-06. Epub 2006 Dec 11. Mol Cell Biol. 2007. PMID: 17158927 Free PMC article.
References
- Svejstrup J Q, Vichi P, Egly J M. Trends Biochem Sci. 1996;21:346–350. - PubMed
- Conaway R C, Conaway J W. J Biol Chem. 1990;265:7559–7563. - PubMed
- Flores O, Lu H, Reinberg D. J Biol Chem. 1992;267:2786–2793. - PubMed
- Luse D S, Jacob G A. J Biol Chem. 1987;262:14990–14997. - PubMed
- Conaway R C, Conaway J W. J Biol Chem. 1988;263:2962–2968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials