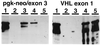Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice - PubMed (original) (raw)
Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice
J R Gnarra et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Inheritance of an inactivated form of the VHL tumor suppressor gene predisposes patients to develop von Hippel-Lindau disease, and somatic VHL inactivation is an early genetic event leading to the development of sporadic renal cell carcinoma. The VHL gene was disrupted by targeted homologous recombination in murine embryonic stem cells, and a mouse line containing an inactivated VHL allele was generated. While heterozygous VHL (+/-) mice appeared phenotypically normal, VHL -/- mice died in utero at 10.5 to 12.5 days of gestation (E10.5 to E12.5). Homozygous VHL -/- embryos appeared to develop normally until E9.5 to E10.5, when placental dysgenesis developed. Embryonic vasculogenesis of the placenta failed to occur in VHL -/- mice, and hemorrhagic lesions developed in the placenta. Subsequent hemorrhage in VHL -/- embryos caused necrosis and death. These results indicate that VHL expression is critical for normal extraembryonic vascular development.
Figures
Figure 1
Targeted disruption of the murine VHL gene in ES cells and mice. (a) Schematic representation of the VHL locus and the VHL targeting vector. Boxes represent the three VHL exons. The open boxes in the targeting vector represent the pgk-neo and pgk-tk selectable markers. The filled boxes in the targeting vector represent a 3-kb _Kpn_I–_Not_I restriction fragment from the 5′ end of the VHL gene and a 3-kb _Nco_I–_Bam_HI restriction fragment from the 3′ end of the gene. The 5′ and 3′ flanking probes used in Southern blotting are indicated. (b) Detection of wild-type and targeted alleles in ES clones 15 and 35 and a representative, untargeted ES clone (−) by Southern blotting. After homologous recombination of the targeting vector in ES cells, _Bam_HI digestion and Southern blotting with the 5′ flanking probe detected in addition to the 5-kb fragment from the wild-type locus, a 4-kb fragment from the targeted locus due to the introduction of a new _Bam_HI site in the targeting vector. Similarly, _Hin_dIII digestion and Southern blotting with the 3′ flanking probe detected the wild-type 8-kb fragment and a 4-kb fragment at the targeted locus, due to the insertion of a _Hin_dIII site in the targeting vector. (c) Southern analysis of _Bam_HI-digested (Upper) or _Hin_dIII (Lower) mouse tail biopsy DNA from a representative intercross litter, showing absence of VHL homozygous mice. The wild-type, 8-kb locus could not be identified in _Hin_dIII digests of DNA extracted from mouse tail biopsies, perhaps because of loss of recognition due to methylation interference.
Figure 2
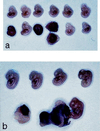
Gross appearance of E12.5 embryos from intercross of VHL +/− mice. (a) Representative litter showing three abnormal embryos of 12 total. VHL −/− embryos are necrotic, and one embryo is shown with a hemorrhagic placenta. (b) Photomicrograph of another representative E12.5 litter showing one abnormal embryo and one embryo of normal appearance that is attached to a hemorrhagic placenta.
Figure 3
Genotyping of abnormal embryos after laser capture microdissection and PCR. The PCR primers tested were specific for VHL exon 1 (reactive only with the wild-type allele), or specific for the targeted locus (the amplicon spans the pgk-neo/exon 3 boundary of the targeted locus). DNA samples were analyzed in triplicate and VHL −/− embryos were identified based on amplification with the targeted allele-specific primers and failure to amplify with VHL exon 1 primers. Group 1, positive control reaction products from PCR using VHL +/− mouse tail biopsy template DNA; group 2, negative control reaction products from PCR reactions containing no added DNA; group 3, PCR reaction products using template DNA isolated from a representative abnormal E10.5 embryo and showing a VHL −/− genotype; group 4, PCR reaction products using template DNA isolated from a portion of maternally derived placenta from a VHL +/− mother; group 5, PCR reaction products using template DNA isolated from a representative normal E10.5 embryo and showing a VHL +/+ genotype.
Figure 4
Histopathology of normal and VHL −/− embryos. (a) Hematoxylin and eosin staining of a normal E10.5 placental labyrinth showing trophoblasts and nucleated erythrocytes in the embryonic vessels and non-nucleated maternal erythrocytes (×300). (b) Hematoxylin and eosin staining of a VHL −/− E10.5 placental labyrinth lacking embryonal vasculogenesis (×300). Note the presence of embryonic vessels with nucleated erythrocytes on the edge of the thin labyrinth that have not invaded the labyrinth (arrows). (c) Hematoxylin and eosin staining of a VHL −/− E11.5 placental showing hemorrhage into the abnormal labyrinth (×150).
Figure 5
Analysis of VHL and VEGF expression in normal and VHL −/− E10.5 embryos. (a) Darkfield photomicrograph of VHL in situ hybridization of a normal embryo using an antisense probe (×30). High levels of VHL expression are seen in the placental labyrinth. (b) Darkfield photomicrograph of VHL in situ hybridization of a VHL −/− embryo using an antisense probe showing no significant hybridization (×30). (c and d) Avidin–biotin complex immunohistochemistry of a normal VHL +/+ E10.5 placenta showing VHL expression in the allantoic mesenchyme (c, ×750) and placental labyrinth trophoblasts (d, ×300). (e) Avidin–biotin complex immunohistochemistry of a normal E10.5 placenta showing VEGF expression in the placental labyrinth trophoblasts (×300). (f) Avidin–biotin complex immunohistochemistry of a VHL −/− E10.5 placenta showing greatly reduced VEGF protein levels in the placental labyrinth trophoblasts (×300).
Similar articles
- Expression of the Von Hippel-Lindau tumor suppressor gene, VHL, in human fetal kidney and during mouse embryogenesis.
Kessler PM, Vasavada SP, Rackley RR, Stackhouse T, Duh FM, Latif F, Lerman MI, Zbar B, Williams BR. Kessler PM, et al. Mol Med. 1995 May;1(4):457-66. Mol Med. 1995. PMID: 8521303 Free PMC article. - Expression of the von Hippel-Lindau disease tumour suppressor gene during human embryogenesis.
Richards FM, Schofield PN, Fleming S, Maher ER. Richards FM, et al. Hum Mol Genet. 1996 May;5(5):639-44. doi: 10.1093/hmg/5.5.639. Hum Mol Genet. 1996. PMID: 8733131 - Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor.
Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Haase VH, et al. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1583-8. doi: 10.1073/pnas.98.4.1583. Proc Natl Acad Sci U S A. 2001. PMID: 11171994 Free PMC article. - Von Hippel-Lindau disease and sporadic renal cell carcinoma.
Zbar B. Zbar B. Cancer Surv. 1995;25:219-32. Cancer Surv. 1995. PMID: 8718521 Review. - [Von Hippel-Lindau disease].
Shuin T, Ashida S, Yao M, Kanno H. Shuin T, et al. Nihon Rinsho. 2000 Jul;58(7):1448-54. Nihon Rinsho. 2000. PMID: 10921322 Review. Japanese.
Cited by
- Hypoxia-mediated rescue of retinal ganglion cells deficient in mitochondrial complex I is independent of the hypoxia-inducible factor pathway.
Warwick AM, Bomze HM, Wang L, Hao Y, Stinnett SS, Gospe SM 3rd. Warwick AM, et al. Sci Rep. 2024 Oct 15;14(1):24114. doi: 10.1038/s41598-024-75916-x. Sci Rep. 2024. PMID: 39406814 Free PMC article. - Hypoxia-inducible factors and cancer.
Calzada MJ, del Peso L. Calzada MJ, et al. Clin Transl Oncol. 2007 May;9(5):278-89. doi: 10.1007/s12094-007-0055-y. Clin Transl Oncol. 2007. PMID: 17525038 Review. - A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors.
Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS. Crabtree JS, et al. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1118-23. doi: 10.1073/pnas.98.3.1118. Proc Natl Acad Sci U S A. 2001. PMID: 11158604 Free PMC article. - Role of the ubiquitin proteasome system in renal cell carcinoma.
Corn PG. Corn PG. BMC Biochem. 2007 Nov 22;8 Suppl 1(Suppl 1):S4. doi: 10.1186/1471-2091-8-S1-S4. BMC Biochem. 2007. PMID: 18047741 Free PMC article. Review. - Impact of reduced uterine perfusion pressure model of preeclampsia on metabolism of placenta, maternal and fetal hearts.
McClements L, Richards C, Patel N, Chen H, Sesperez K, Bubb KJ, Karlstaedt A, Aksentijevic D. McClements L, et al. Sci Rep. 2022 Jan 21;12(1):1111. doi: 10.1038/s41598-022-05120-2. Sci Rep. 2022. PMID: 35064159 Free PMC article.
References
- Linehan W M, Lerman M I, Zbar B. J Am Med Assoc. 1995;273:564–570. - PubMed
- Gnarra J R, Duan D R, Weng Y, Humphrey J S, Chen D Y T, Lee S, Pause A, Dudley C F, Latif F, Kuzmin I, Schmidt L, Duh F-M, Lerman M I, Zbar B, Klausner R D, Linehan W M. Biochim Biophys Acta. 1996;1242:201–210. - PubMed
- Duan D R, Pause A, Burgess W H, Aso T, Chen D Y T, Garrett K P, Conaway R C, Conaway J W, Linehan W M, Klausner R D. Science. 1995;269:1402–1406. - PubMed
- Aso T, Lane W S, Conaway J W, Conaway R C. Science. 1995;269:1439–1443. - PubMed
- Kibel A, Iliopoulos O, DeCaprio J A, Kaelin W G. Science. 1995;269:1444–1446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases