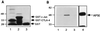Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein - PubMed (original) (raw)
Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein
Y Zhang et al. Proc Natl Acad Sci U S A. 1997.
Abstract
CTLA-4 plays a critical role in regulating the immune response. It is mainly located in cytoplasmic vesicles and is expressed only transiently on the surface after T cell activation. In this study, we demonstrate that CTLA-4 is associated with AP50, the medium chain of the clathrin-associated coated pit adaptor protein complex AP2. In a yeast two-hybrid screen, three individual cDNA clones that encode mouse AP50 were isolated, all of which can interact specifically with the cytoplasmic domain of mouse CTLA-4, but not with the cytoplasmic domain of mouse CD28. We have shown that CTLA-4 can bind specifically to AP50 when CTLA-4 and AP50 are cotransfected into human 293T cells. A Y201 to F201 mutation in the YVKM intracellular localization motif of the CTLA-4 cytoplasmic domain significantly diminished its binding to AP50. We also found that AP50 bound to a CTLA-4 peptide containing unphosphorylated Y201 but not to a peptide containing phosphorylated Y201. Conversely, the p85 subunit of phosphatidylinositol 3-kinase and, to a lesser extent, protein tyrosine phosphatase SYP (SHP-2) and SHP (SHP-1) bind only to the CTLA-4 peptide containing phosphorylated Y201. Therefore, the phosphorylation status of Y201 in the CTLA-4 cytoplasmic domain determines the binding specificity of CTLA-4. These results suggest that AP50 and the coated pit adaptor complex AP2 may play an important role in regulating the intracellular trafficking and function of CTLA-4.
Figures
Figure 1
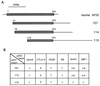
Yeast two-hybrid cloning of murine AP50. (A) Schematic diagram of the cDNA clones encoding murine AP50. The cDNA inserts from clones 107, 114, and 119 are 1.6, 1.6, and 2.0 kb, respectively. (B) Yeast specificity test. Plasmids (pAS2-CYH) encoding the GAL4 DNA-binding domain fused to the proteins indicated on the top of each column were transfected into yeast strain Y187 and mated with yeast strain Y190 containing the plasmids (pACT) encoding the GAL4 activation domain fused with AP50 proteins. The yeast diploids which contain both plasmids were picked and tested in the β-galactosidase assay. RB, retinoblastoma protein; n.d., not determined.
Figure 2
Interaction between CTLA-4 and AP50 in vitro. (A) Expression of CTLA-4 cytoplasmic domain as a GST-fusion protein. GST-CTLA-4 (lane 1), GST-c-Jun (lane 2), and GST (lane 3) were separated by SDS/10% PAGE and stained with Coomassie blue. (B) Binding of HA-tagged-AP50 to GST-fusion proteins. Lysates of 293T cells containing HA-AP50 were incubated with immobilized GST protein (lane 1), GST-CTLA-4 (lane 2), or GST-c-Jun (lane 3). Bound HA-AP50 was eluted from glutathione-agarose by boiling in SDS sample buffer, separated by SDS/10% PAGE, and transferred onto PVDF membrane. HA-AP50 was detected by blotting with an anti-HA antibody. HA-AP50 in the 293T cell lysate was also immunoprecipitated using anti-HA-coated protein G agarose (lane 4).
Figure 3
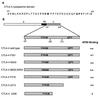
CTLA-4 mutants and their interaction with AP50. (A) Amino acid sequence of the CTLA-4 cytoplasmic domain. Boldface letters indicate the tyrosine-containing motifs that are conserved between CD28 and CTLA-4. (B) List of point mutations and deletion mutations in the cytoplasmic domain of CTLA-4. Domains: E, external; TM, transmembrane; C, cytoplasmic. The binding capacities of wild-type CTLA-4 and the mutants to AP50 are summarized on the basis of representative data shown in Fig. 4 and other results that are not shown.
Figure 4
Coimmunoprecipitation of AP50 with CTLA-4 mutants from 293T cells. Lysates of 293T cells cotransfected with CTLA-4 and AP50 were immunoprecipitated with anti-CTLA-4 antibody 9H10. The presence of AP50 in the immunoprecipitates was detected by immunoblotting with anti-HA. The membranes in A and B Upper were stripped and reblotted with polyclonal anti-CTLA-4 antibody Q20 (Lower).
Figure 5
Interaction between CD28 and CTLA-4 peptides and AP50. (A) Binding of phosphorylated or unphosphorylated CD28 and CTLA-4 peptides with AP50. Lysates of HA-AP50 transfected 293T cells were incubated with Affi-Gel (lane 1) or Affi-Gel-coupled unphosphorylated CD28 peptide (lane 2) or phosphorylated CD28 peptide (lane 3) or unphosphorylated CTLA-4 peptide (lane 5) or phosphorylated CTLA-4 peptide (lane 7). The Affi-Gel-coupled phosphorylated CD28 (lane 4) and unphosphorylated CTLA-4 peptide (lane 6) and phosphorylated CTLA-4 peptide (lane 8) were also first digested with alkaline phosphatase before incubation with AP50. (B) Competition of AP50 binding to Affi-Gel-coupled CTLA-4 peptide. Affi-Gel (lane 1) or CTLA-4 peptide-coupled Affi-Gel (lanes 2–8) was incubated with equal amounts of HA-AP50 in the presence of the indicated amounts of unphosphorylated (lanes 3–5) and phosphorylated (lanes 6–8) CTLA-4 peptides. The Affi-Gel-bound AP50 was analyzed as described for Fig. 2. The total amount of AP50 in each binding reaction is shown in A, lane 0, and B, lane 9, by immunoprecipitation with anti-HA antibody.
Figure 6
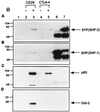
Interaction between CD28 and CTLA-4 peptides with SH2 domain-containing proteins. (A) Jurkat cell lysates were incubated with Affi-Gel (lane 1), Affi-Gel-coupled unphosphorylated CD28 peptide (lane 2), phosphorylated CD28 peptide (lane 3), unphosphorylated CTLA-4 peptide (lane 4), or phosphorylated CTLA-4 peptide (lane 5). Jurkat cell lysates were also immunoprecipitated with anti-SHP (lane 6) and anti-SYP (lane 7) polyclonal antibodies. Proteins bound to the Affi-Gel were separated by SDS/PAGE and Western blotted with anti-SYP polyclonal antibody. The same PVDF membrane was stripped and blotted again with anti-SHP (B), anti-p85 (C), and anti-Grb-2 (D). A lysate of approximately 3 × 106 Jurkat cells was used for each binding reaction.
Similar articles
- Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression.
Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander Heiden MG, Thompson CB. Chuang E, et al. J Immunol. 1997 Jul 1;159(1):144-51. J Immunol. 1997. PMID: 9200449 - Study of the interaction of the medium chain mu 2 subunit of the clathrin-associated adapter protein complex 2 with cytotoxic T-lymphocyte antigen 4 and CD28.
Follows ER, McPheat JC, Minshull C, Moore NC, Pauptit RA, Rowsell S, Stacey CL, Stanway JJ, Taylor IW, Abbott WM. Follows ER, et al. Biochem J. 2001 Oct 15;359(Pt 2):427-34. doi: 10.1042/0264-6021:3590427. Biochem J. 2001. PMID: 11583591 Free PMC article. - Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation.
Bradshaw JD, Lu P, Leytze G, Rodgers J, Schieven GL, Bennett KL, Linsley PS, Kurtz SE. Bradshaw JD, et al. Biochemistry. 1997 Dec 16;36(50):15975-82. doi: 10.1021/bi971762i. Biochemistry. 1997. PMID: 9398332 - Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2.
Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Shiratori T, et al. Immunity. 1997 May;6(5):583-9. doi: 10.1016/s1074-7613(00)80346-5. Immunity. 1997. PMID: 9175836
Cited by
- Mechanistic Insights into the Inhibition of a Common CTLA-4 Gene Mutation in the Cytoplasmic Domain.
Xu J, Zhang Y, Shen L, Du L, Xue H, Wu B, OuYang B. Xu J, et al. Molecules. 2024 Mar 16;29(6):1330. doi: 10.3390/molecules29061330. Molecules. 2024. PMID: 38542966 Free PMC article. - Harnessing the immune system by targeting immune checkpoints: Providing new hope for Oncotherapy.
Yu L, Sun M, Zhang Q, Zhou Q, Wang Y. Yu L, et al. Front Immunol. 2022 Sep 8;13:982026. doi: 10.3389/fimmu.2022.982026. eCollection 2022. Front Immunol. 2022. PMID: 36159789 Free PMC article. Review. - Current Understanding of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Signaling in T-Cell Biology and Disease Therapy.
Kim GR, Choi JM. Kim GR, et al. Mol Cells. 2022 Aug 31;45(8):513-521. doi: 10.14348/molcells.2022.2056. Epub 2022 Jul 27. Mol Cells. 2022. PMID: 35950451 Free PMC article. Review. - A MET-PTPRK kinase-phosphatase rheostat controls ZNRF3 and Wnt signaling.
Kim M, Reinhard C, Niehrs C. Kim M, et al. Elife. 2021 Sep 30;10:e70885. doi: 10.7554/eLife.70885. Elife. 2021. PMID: 34590584 Free PMC article. - Regulation of CTLA-4 recycling by LRBA and Rab11.
Janman D, Hinze C, Kennedy A, Halliday N, Waters E, Williams C, Rowshanravan B, Hou TZ, Minogue S, Qureshi OS, Sansom DM. Janman D, et al. Immunology. 2021 Sep;164(1):106-119. doi: 10.1111/imm.13343. Epub 2021 Jun 6. Immunology. 2021. PMID: 33960403 Free PMC article.
References
- Linsley P S, Ledbetter J A. Annu Rev Immunol. 1993;11:191–212. - PubMed
- June C H, Bluestone J A, Nadler L M, Thompson C B. Immunol Today. 1994;15:321–331. - PubMed
- Allison J P. Curr Opin Immunol. 1994;6:414–419. - PubMed
- Brunet J F, Denizot F, Luciani M F, Roux-Dosseto M, Suzan M, Mattei M F, Golstein P. Nature (London) 1987;328:267–270. - PubMed
- Harper K, Balzano C, Rouvier E, Mattei M G, Luciani M F, Golstein P. J Immunol. 1991;147:1037–1044. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials