Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice - PubMed (original) (raw)
Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice
M B Alimzhanov et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The tumor necrosis factor (TNF) family cytokines lymphotoxin (LT) alpha and LTbeta form heterotrimers that are expressed on the surface of activated lymphocytes and natural killer cells; LTalpha homotrimers can be secreted as well. Mice with a disrupted LTalpha gene lack lymph nodes (LN), Peyer's patches (PP), and follicular dendritic cell (FDC) networks and reveal profound defects of the splenic architecture. However, it is unclear which of these abnormalities is the result of the absence in LTalpha homotrimers or LTalphabeta heterotrimers. To distinguish between these two possibilities, a mouse strain deficient in LTbeta was created employing Cre/loxP-mediated gene targeting. Mice deficient in LTbeta reveal severe defects in organogenesis of the lymphoid system similar to those of LTalpha-/- mice, except that mesenteric and cervical LN are present in most LTbeta-deficient mice. Both LTbeta- and LTalpha-deficient mice show significant lymphocytosis in the circulation and peritoneal cavity and lymphocytic infiltrations in lungs and liver. After immunization, PNA-positive B cell clusters were detected in the splenic white pulp of LTbeta-deficient mice, but FDC networks were severely underdeveloped. Collectively, these results indicate that LTalpha can signal independently from LTbeta in the formation of PNA-positive foci in the spleen, and especially in the development of mesenteric and cervical LN.
Figures
Figure 1
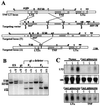
Generation of LTβΔ/Δ mice. (A) Targeting strategy used for inactivation of the mouse LTβ gene. B, _Bam_HI; H, _Hin_dIII; K, _Kpn_I; M, _Mlu_I; N, _Nde_I. (B) Southern blot analysis of genomic DNA from targeted ES cells and from mouse tail biopsies. (C) Northern blot analysis of total RNA extracted from thymocytes or ConA-activated splenocytes derived from LTβ+/+, LTβ+/Δ, and LTβΔ/Δ mice. 18S RNA was stained with methylene blue (loading control).
Figure 2
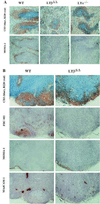
Peripheral lymphoid organs in LTβΔ/Δ mice as compared with LTα−/− mice (11). (A) Defective spleen organization in LTβΔ/Δ and LTα−/− mice. Splenic cryosections of 6- to 8-week-old mice were processed to detect the distribution of T cells (CD3, blue) and B cells (B220, red) or metallophilic macrophages (MOMA-1). Original magnification, ×100. (B) Immunohistological analysis of mesenteric LN from LTβΔ/Δ mice. Serial sections were labeled with anti-CD3 (blue)/anti-B220 (red), anti-MOMA-1, anti-MAdCAM-1, or anti-FDC-M1 antibody.
Figure 3
Lymphocytic infiltrations in lungs of LTβΔ/Δ and LTα−/− mice. Serial sections of frozen organs from 8-week-old mice were labeled with anti-CD4, anti-CD8, or anti-B220 antibody. Original magnification, ×100.
Figure 4
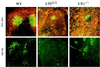
Impaired FDC network development and germinal center formation in spleens of LTβΔ/Δ and LTα−/− mice. Eight-week-old mice were immunized intraperitoneally with SRBC, and 10 days later spleens were removed. Immunofluorescence microscopy was performed on cryosections of spleens to detect formation of germinal centers (PNA-FITC IgM-Texas Red) and development of FDC networks (FDC-M2). Original magnification, ×100.
Similar articles
- Membrane lymphotoxin contributes to anti-leishmanial immunity by controlling structural integrity of lymphoid organs.
Wilhelm P, Riminton DS, Ritter U, Lemckert FA, Scheidig C, Hoek R, Sedgwick JD, Körner H. Wilhelm P, et al. Eur J Immunol. 2002 Jul;32(7):1993-2003. doi: 10.1002/1521-4141(200207)32:7<1993::AID-IMMU1993>3.0.CO;2-F. Eur J Immunol. 2002. PMID: 12115620 - TNF and lymphotoxin beta cooperate in the maintenance of secondary lymphoid tissue microarchitecture but not in the development of lymph nodes.
Kuprash DV, Alimzhanov MB, Tumanov AV, Anderson AO, Pfeffer K, Nedospasov SA. Kuprash DV, et al. J Immunol. 1999 Dec 15;163(12):6575-80. J Immunol. 1999. PMID: 10586051 - A role for tumor necrosis factor receptor type 1 in gut-associated lymphoid tissue development: genetic evidence of synergism with lymphotoxin beta.
Koni PA, Flavell RA. Koni PA, et al. J Exp Med. 1998 Jun 15;187(12):1977-83. doi: 10.1084/jem.187.12.1977. J Exp Med. 1998. PMID: 9625757 Free PMC article. - Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting.
Tumanov AV, Grivennikov SI, Shakhov AN, Rybtsov SA, Koroleva EP, Takeda J, Nedospasov SA, Kuprash DV. Tumanov AV, et al. Immunol Rev. 2003 Oct;195:106-16. doi: 10.1034/j.1600-065x.2003.00071.x. Immunol Rev. 2003. PMID: 12969314 Review. - Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers.
Matsumoto M, Fu YX, Molina H, Chaplin DD. Matsumoto M, et al. Immunol Rev. 1997 Apr;156:137-44. doi: 10.1111/j.1600-065x.1997.tb00965.x. Immunol Rev. 1997. PMID: 9176705 Review.
Cited by
- Lymphotoxin in physiology of lymphoid tissues - Implication for antiviral defense.
Koroleva EP, Fu YX, Tumanov AV. Koroleva EP, et al. Cytokine. 2018 Jan;101:39-47. doi: 10.1016/j.cyto.2016.08.018. Epub 2016 Sep 9. Cytokine. 2018. PMID: 27623349 Free PMC article. Review. - Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation.
Heesters BA, Chatterjee P, Kim YA, Gonzalez SF, Kuligowski MP, Kirchhausen T, Carroll MC. Heesters BA, et al. Immunity. 2013 Jun 27;38(6):1164-75. doi: 10.1016/j.immuni.2013.02.023. Epub 2013 Jun 13. Immunity. 2013. PMID: 23770227 Free PMC article. - Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis.
Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. Lee BJ, et al. J Virol. 2000 Mar;74(6):2786-92. doi: 10.1128/jvi.74.6.2786-2792.2000. J Virol. 2000. PMID: 10684295 Free PMC article. - Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells.
Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Gorelik L, et al. J Exp Med. 2003 Sep 15;198(6):937-45. doi: 10.1084/jem.20030789. J Exp Med. 2003. PMID: 12975458 Free PMC article. - Role of the Lymphotoxin/LIGHT System in the Development and Maintenance of Reticular Networks and Vasculature in Lymphoid Tissues.
Lu TT, Browning JL. Lu TT, et al. Front Immunol. 2014 Feb 11;5:47. doi: 10.3389/fimmu.2014.00047. eCollection 2014. Front Immunol. 2014. PMID: 24575096 Free PMC article. Review.
References
- Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. - PubMed
- Muller U, Jongeneel C V, Nedospasov S A, Fisher Lindahl K, Steinmetz M. Nature (London) 1987;325:265–267. - PubMed
- Browning J L, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow E P, Hession C, O’Brine-Greco B, Foley S F, Ware C F. Cell. 1993;72:847–856. - PubMed
- Ware C F, Crowe P D, Grayson M H, Androlewicz M J, Browning J L. J Immunol. 1992;149:3881–3888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous