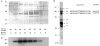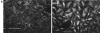Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins - PubMed (original) (raw)
Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins
D J Mackay et al. J Cell Biol. 1997.
Abstract
The small GTPases Rho and Rac regulate actin filament assembly and the formation of integrin adhesion complexes to produce stress fibers and lamellipodia, respectively, in mammalian cells. Although numerous candidate effectors that might mediate these responses have been identified using the yeast two-hybrid and affinity purification techniques, their cellular roles remain unclear. We now describe a biological assay that allows components of the Rho and Rac signaling pathways to be identified. Permeabilization of serum-starved Swiss 3T3 cells with digitonin in the presence of guanosine 5'-O-(3-thiotriphosphate) (GTPgammaS) induces both actin filament and focal adhesion complex assembly through activation of endogenous Rho and Rac. These responses are lost when GTPgammaS is added 6 min after permeabilization, but can be reconstituted using concentrated cytosolic extracts. We have achieved a 10,000-fold purification of the activity present in pig brain cytosol and protein sequence analysis shows it to contain moesin. Using recombinant proteins, we show that moesin and its close relatives ezrin and radixin can reconstitute stress fiber assembly, cortical actin polymerization and focal complex formation in response to activation of Rho and Rac.
Figures
Figure 5
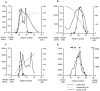
Purification of the active component from pig brain cytosol. (A) Cibachrome blue 3GA: a step-eluted fraction from fast-flow Q-Sepharose, diluted to 50 mM salt, was passed over a 40 ml Cibachrome blue 3GA column, washed extensively, and eluted as shown in a continuous 25-300 μS salt gradient. Protein was monitored in line by absorbence at 280 nm, and salt concentration by conductivity, as shown. The activities of column fractions were assayed and plotted (far left ordinate axis) and the two activity peaks were pooled. (B) Phenyl–Sepharose: pooled fractions from A were applied to a 15-ml phenyl–Sepharose column and proteins eluted with a 300–25 μS salt gradient. (C) Q-Sepharose: pooled active fractions from B were diluted to 20 mM salt and chromatographed on a 5-ml Q-Sepharose column. Activity was eluted in a salt gradient to 200 μS. (D) 200 μl of the peak fraction from C was applied to a Bio-Silect gel filtration column with a rated separation range of 100–5 kD. The elution positions of molecular weight standards are indicated.
Figure 6
Moesin copurifies with GTPγS-dependent biological activity in permeabilized cells. (A) Silver-stained 10% SDS-PAGE using 10-μl aliquots from fractions 28–35 of the gel-filtration column shown in Fig. 5_D._ Relative activities of the gel-filtered fractions are noted below the corresponding lanes of the blot. (B) 22 μg of fraction 22, the most active from the Q-Sepharose column shown in Fig. 5_c_, was electrophoresed on 10% SDS-PAGE and electroblotted onto PVDF, and the major protein bands A–F were excised and microsequenced. The sequences obtained are shown. (C) Proteins from a parallel gel to A were transferred to PVDF and Western blotted with a polyclonal anti-ERM antibody.
Figure 9
F-actin binding to moesin. (a and b) F-actin binding to recombinant and purified pig brain moesin: lane 1, 35 ng recombinant moesin; lane 2, 125 ng of fraction 22 from Q-Sepharose column, estimated to contain 30 ng moesin. (A) immunoreactivity with monoclonal anti-moesin antibody; (B) F-actin binding using [32P]ATP labeled F-actin nitrocellulose overlay assay. (C) Loss of moesin from permeabilized cells. Coverslips were incubated under the following conditions: lanes 1 and 2, mock-permeabilized for 6 min in 60 μl DK without digitonin; lanes 3 and 4, permeabilized for 6 min in 60 μl DK containing 0.003% digitonin. Cell supernatants (lanes 1 and 3) and residues (lanes 2 and 4) were prepared and proteins electrophoresed on 10% SDS-PAGE gels, transferred to PVDF, and blotted with a polyclonal antibody against ERM proteins. (D and E) F-actin binding of moesin in Swiss 3T3 cells. Lane 1, 75 ng recombinant moesin; lane 2, total SDS lysate; lane 3, total SDS lysate from cells that had been first incubated for 6 min in 60 μl DK with 0.003% digitonin. Samples equivalent to half coverslips of cells were electrophoresed on 10% SDS-PAGE gels, transferred to PVDF membranes, and then probed with (D) polyclonal anti-ERM antibody (E) [32P]ATP-labeled F-actin.
Figure 1
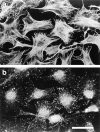
Permeabilization of quiescent Swiss 3T3 cells in the presence of GTPγS. (A) Permeabilization (protocol 1) was performed in the absence of stimulus (left) or in the presence of 50 μM GTPγS (right). Cellular F-actin was visualized using rhodamine-conjugated phalloidin. (B) Permeabilization (protocol 1) was performed in the absence of stimulus (a and b), in the presence of 50 μM GTPγS (c and d); 50 μM GTPγS with 0.1 nM C3 transferase (e and f); 50 μM GTPγS with 1 nM N17Rac (g and h). After 20 min at 37°C, cells were fixed and F-actin visualized with rhodamine-phalloidin (a, c, e, and g) and vinculin visualized with a monoclonal antibody (b, d, f, and h). In c and d, arrowheads show termini of bundled actin filaments decorated with focal adhesions, while arrows mark regions of peripheral actin polymerization decorated with linear arrays of focal complexes. Bars: (A) 150 μm; (B) 30 μm.
Figure 1
Permeabilization of quiescent Swiss 3T3 cells in the presence of GTPγS. (A) Permeabilization (protocol 1) was performed in the absence of stimulus (left) or in the presence of 50 μM GTPγS (right). Cellular F-actin was visualized using rhodamine-conjugated phalloidin. (B) Permeabilization (protocol 1) was performed in the absence of stimulus (a and b), in the presence of 50 μM GTPγS (c and d); 50 μM GTPγS with 0.1 nM C3 transferase (e and f); 50 μM GTPγS with 1 nM N17Rac (g and h). After 20 min at 37°C, cells were fixed and F-actin visualized with rhodamine-phalloidin (a, c, e, and g) and vinculin visualized with a monoclonal antibody (b, d, f, and h). In c and d, arrowheads show termini of bundled actin filaments decorated with focal adhesions, while arrows mark regions of peripheral actin polymerization decorated with linear arrays of focal complexes. Bars: (A) 150 μm; (B) 30 μm.
Figure 2
Recombinant Rho stimulates formation of focal adhesions and stress fibers in permeabilized cells. Cells were permeabilized (protocol 1) in the presence of 1 μM recombinant V14Rho. (a) Phalloidin staining for F-actin; (b) monoclonal antibody against vinculin. Bar, 30 μm.
Figure 4
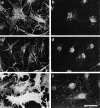
Reconstitution of Rho-induced effects after extended permeabilization using a pig brain cytosolic extract. Cells were permeabilized according to protocol 2 (i.e., permeabilized for 6 min in the presence of digitonin before addition of stimulus; see Materials and Methods) in the presence of (a and b) 25 μg/ml V14Rho, (c and d) 2 mg/ml pig brain extract, (e and f) 25 μg/ml V14Rho plus 2 mg/ml pig brain extract. F-actin in the permeabilized cells was visualized using rhodamine-conjugated phalloidin (a, c, and e) and focal adhesions with anti-vinculin antiserum (b, d, and f). Bar, 30 μm.
Figure 3
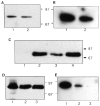
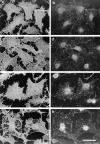
Retention of cytosolic and cytoskeletal markers after permeabilization. (a) LDH activity, (b) vinculin visualized by Western blot, and (c) Rho visualized after ADP ribosylation with C3 transferase and 32P-NAD. (a) Coverslips were incubated for 20 min at 37°C in 50 μl vol ± 0.003% digitonin, or for 6 min at room temperature in 60 μl vol containing either 0.003% digitonin or 0.2% Triton X-100. Samples were assayed for LDH in the presence of 0.2% Triton X-100. Data are mean ± SD from four independent determinations. (b) Coverslips were incubated for 6 min at room temperature in 60 μl buffer containing no detergent, 0.003% digitonin or 0.2% (vol/vol) Triton X-100, and supernatants and pellets were recovered. Proteins were electrophoresed on 12% SDS-PAGE and transferred to PVDF, and vinculin was visualized using a monoclonal anti-vinculin antibody. (c) Cell supernatants and pellets were prepared as in b, and endogenous Rho determined as described in Materials and Methods. Positions of molecular weight standards (kD) are marked.
Figure 3
Retention of cytosolic and cytoskeletal markers after permeabilization. (a) LDH activity, (b) vinculin visualized by Western blot, and (c) Rho visualized after ADP ribosylation with C3 transferase and 32P-NAD. (a) Coverslips were incubated for 20 min at 37°C in 50 μl vol ± 0.003% digitonin, or for 6 min at room temperature in 60 μl vol containing either 0.003% digitonin or 0.2% Triton X-100. Samples were assayed for LDH in the presence of 0.2% Triton X-100. Data are mean ± SD from four independent determinations. (b) Coverslips were incubated for 6 min at room temperature in 60 μl buffer containing no detergent, 0.003% digitonin or 0.2% (vol/vol) Triton X-100, and supernatants and pellets were recovered. Proteins were electrophoresed on 12% SDS-PAGE and transferred to PVDF, and vinculin was visualized using a monoclonal anti-vinculin antibody. (c) Cell supernatants and pellets were prepared as in b, and endogenous Rho determined as described in Materials and Methods. Positions of molecular weight standards (kD) are marked.
Figure 7
Recombinant moesin reconstitutes GTPγS- induced effects. Cells were permeabilized according to protocol 2, and incubated with 50 nM (saturating) recombinant moesin and 50 μM GTPγS (a–f); additionally, in c and d 0.1 nM C3 was present and in e and f 1 nM N17rac. The cells shown in g and h were incubated with 50 nM truncated moesin and 50 μM GTPγS. (a, c, e, and g) Phalloidin staining for F-actin; (b, d, f, and h) monoclonal antibody against vinculin. Bar, 30 μm.
Figure 8
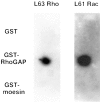
Moesin does not interact directly with Rho or Rac. 5 μg aliquots of GST, GST-RhoGAP, and GST-moesin were spotted onto nitrocellulose and the filter blocked with blocking buffer. The filter was then incubated with 0.1 μg of [γ32P]GTP-loaded Rho or Rac and subjected to autoradiography.
Similar articles
- Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization.
Machesky LM, Hall A. Machesky LM, et al. J Cell Biol. 1997 Aug 25;138(4):913-26. doi: 10.1083/jcb.138.4.913. J Cell Biol. 1997. PMID: 9265656 Free PMC article. - ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts.
Norman JC, Jones D, Barry ST, Holt MR, Cockcroft S, Critchley DR. Norman JC, et al. J Cell Biol. 1998 Dec 28;143(7):1981-95. doi: 10.1083/jcb.143.7.1981. J Cell Biol. 1998. PMID: 9864369 Free PMC article. - Ras-related GTPases and the cytoskeleton.
Hall A. Hall A. Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review. - Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes.
Ivetic A, Ridley AJ. Ivetic A, et al. Immunology. 2004 Jun;112(2):165-76. doi: 10.1111/j.1365-2567.2004.01882.x. Immunology. 2004. PMID: 15147559 Free PMC article. Review.
Cited by
- Interaction of proteins identified in human thyroid cells.
Pietsch J, Riwaldt S, Bauer J, Sickmann A, Weber G, Grosse J, Infanger M, Eilles C, Grimm D. Pietsch J, et al. Int J Mol Sci. 2013 Jan 9;14(1):1164-78. doi: 10.3390/ijms14011164. Int J Mol Sci. 2013. PMID: 23303277 Free PMC article. - Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function.
Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Simonovic I, et al. Infect Immun. 2001 Sep;69(9):5679-88. doi: 10.1128/IAI.69.9.5679-5688.2001. Infect Immun. 2001. PMID: 11500444 Free PMC article. - Nuclear import of Xenopus egg extract components into cultured cells for reprogramming purposes: a case study on goldfish fin cells.
Chênais N, Lorca T, Morin N, Guillet B, Rime H, Le Bail PY, Labbé C. Chênais N, et al. Sci Rep. 2019 Feb 27;9(1):2861. doi: 10.1038/s41598-019-39500-y. Sci Rep. 2019. PMID: 30814557 Free PMC article. - Expression of ezrin correlates with malignant phenotype of lung cancer, and in vitro knockdown of ezrin reverses the aggressive biological behavior of lung cancer cells.
Li Q, Gao H, Xu H, Wang X, Pan Y, Hao F, Qiu X, Stoecker M, Wang E, Wang E. Li Q, et al. Tumour Biol. 2012 Oct;33(5):1493-504. doi: 10.1007/s13277-012-0400-9. Epub 2012 Apr 20. Tumour Biol. 2012. PMID: 22528947 - The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell-cell contact through activation of Rho-like GTPases.
Sadekova S, Lamarche-Vane N, Li X, Beauchemin N. Sadekova S, et al. Mol Biol Cell. 2000 Jan;11(1):65-77. doi: 10.1091/mbc.11.1.65. Mol Biol Cell. 2000. PMID: 10637291 Free PMC article.
References
- Aktories K, Just I. In vitro ADP-ribosylation of rho by bacterial ADP-ribosyltransferases. Methods Enzymol. 1995;256:184–195. - PubMed
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target of rho as the serine-threonine kinase protein kinase N. Science (Wash DC) 1996;271:648–650. - PubMed
- Amieva MR, Furthmayr H. Subcellular localization of moesin in dynamic filopodia, retraction fibers, and other structures involved in substrate exploration, attachment and cell-cell contacts. Exp Cell Res. 1995;219:180–196. - PubMed
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous