Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines - PubMed (original) (raw)
. 1997 Aug 15;90(4):1594-9.
Affiliations
- PMID: 9269778
- PMCID: PMC2778580
Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines
P Brossart et al. Blood. 1997.
Abstract
Several recent studies have shown that dendritic cells (DC) pulsed with soluble proteins can present peptide epitopes derived from these exogenous antigens on major histocompatability complex (MHC) class I molecules and induce an antigen-specific cytotoxic T lymphocyte (CTL) response. We provide evidence here that DC use macropinocytosis to capture soluble antigens that are then presented on MHC class I molecules. The presentation of an epitope derived from soluble ovalbumin was transporter associated with antigen presentation (TAP)-dependent, brefeldin A-sensitive, blocked by inhibitors of proteasomes, and resistant to chloroquine. These data suggest that exogenous antigens access the cytosol of DC and are proccessed for presentation via the same pathway described for conventional MHC class I-restricted cytosolic antigens. Proinflammatory mediators such as tumor necrosis factor-alpha (TNF-alpha) and lipopolysaccharide (LPS) reduced the efficiency of ovalbumin presentation via this pathway. This reduced presentation was not due to impaired expression of class I molecules because these substances upregulated the cell surface expression of Kb-molecules comparable to levels induced by interferon-gamma (IFN-gamma) treatment. The addition of IFN-gamma increased ovalbumin presentation even in the presence of TNF-alpha or LPS. These results show that DC might be involved in the cross-priming phenomenon. This could offer the immune system an additional pathway for effective priming of cytotoxic T cells and provide the possibility to activate both CD4 and CD8 T-cell responses.
Figures
Fig 1
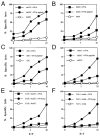
Presentation of exogenous soluble ovalbumin on MHC class I molecules by DC and macrophages. DC (bmDC and sDC) and macrophages (bmM and sMac) isolated from bone marrow or spleens of C57BL/6 and TAP1° mice were incubated for 16 hours with 2 mg/mL ovalbumin or pulsed for 2 hours with synthetic OVA peptide (1 _μ_mol/L) and used as targets in a standard 51Cr-release assay.
Fig 2
Kinetics of delivery of antigenic peptides derived from exogenous antigens into class I presentation pathway of DC. bmDC were cultured in the presence of 2 mg/mL ovalbumin and 20 ng/mL GM-CSF for different time periods. Lysis of bmDC pulsed with OVA protein by the OVA-specific CTL clone B3 was assessed in a standard 51Cr-release assay. DC pulsed with 1 _μ_mol/L OVA peptide (DC + OVA peptide) or untreated DC (DC) were included as a control.
Fig 3
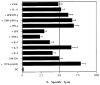
The presentation of soluble antigens on MHC class I molecules by DC is altered by proinflammatory mediators. bmDC grown in media containing 20 ng/mL GM-CSF were cultured in the presence or absence of TNF-α (50 ng/mL), IL-12 (50 ng/mL), IL-7 (50 ng/mL), IL-6 (100 ng/mL), IL-4 (20 ng/mL), IFN-γ (100 U/mL), LPS (10 _μ_g/mL), or Flt3L (100 ng/mL). After 24 hours of incubation, 2 mg/mL of soluble ovalbumin was added and 16 hours later the presentation of ovalbumin by bmDC to the OVA-specific B3 clone was analyzed in a standard 51Cr-release assay. DC pulsed with 1 _μ_mol/L OVA peptide (+OVA peptide) or untreated DC (DC) were included as a control. CTL were added at an E:T ratio of 10:1. The assay was conducted in quadriplicates and error bars show the means and standard deviation.
Fig 4
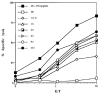
In vivo induction of OVA-specific CTL by DC. DC isolated from bone marrow (bmDC) or spleens (sDC) and splenic macrophages (sMac) from C57BL/6 mice were pulsed with soluble ovalbumin and 4 × 105 DC were injected intraperitoneally on day 0 and 7 into C57BL/6 mice. Splenocytes from immunized mice were harvested on day 14 and stimulated with syngeneic splenocytes pulsed with 10 _μ_mol/L OVA peptide. The primed CTL were then assayed for their ability to lyse E.G7 cells, transfectants expressing the OVA peptide, or EL-4 tumor cells either pulsed with 1 _μ_mol/L OVA peptide or left unpulsed. Mice injected with saline or unpulsed DC gave no response. (■) EL-4 + OVA peptide; (●) EL-4; (◇) EG.7.
Similar articles
- The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens.
Haicheur N, Bismuth E, Bosset S, Adotevi O, Warnier G, Lacabanne V, Regnault A, Desaymard C, Amigorena S, Ricciardi-Castagnoli P, Goud B, Fridman WH, Johannes L, Tartour E. Haicheur N, et al. J Immunol. 2000 Sep 15;165(6):3301-8. doi: 10.4049/jimmunol.165.6.3301. J Immunol. 2000. PMID: 10975847 - Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells.
Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Norbury CC, et al. Eur J Immunol. 1997 Jan;27(1):280-8. doi: 10.1002/eji.1830270141. Eur J Immunol. 1997. PMID: 9022030 - Spotlight on TAP and its vital role in antigen presentation and cross-presentation.
Mantel I, Sadiq BA, Blander JM. Mantel I, et al. Mol Immunol. 2022 Feb;142:105-119. doi: 10.1016/j.molimm.2021.12.013. Epub 2021 Dec 29. Mol Immunol. 2022. PMID: 34973498 Free PMC article. Review. - Function of the transport complex TAP in cellular immune recognition.
Abele R, Tampé R. Abele R, et al. Biochim Biophys Acta. 1999 Dec 6;1461(2):405-19. doi: 10.1016/s0005-2736(99)00171-6. Biochim Biophys Acta. 1999. PMID: 10581370 Review.
Cited by
- New developments in dendritic cell-based vaccinations: RNA translated into clinics.
Grünebach F, Müller MR, Brossart P. Grünebach F, et al. Cancer Immunol Immunother. 2005 Jun;54(6):517-25. doi: 10.1007/s00262-004-0605-x. Epub 2005 Jan 27. Cancer Immunol Immunother. 2005. PMID: 15838706 Free PMC article. Review. - IFN-gamma enables cross-presentation of exogenous protein antigen in human Langerhans cells by potentiating maturation.
Matsuo M, Nagata Y, Sato E, Atanackovic D, Valmori D, Chen YT, Ritter G, Mellman I, Old LJ, Gnjatic S. Matsuo M, et al. Proc Natl Acad Sci U S A. 2004 Oct 5;101(40):14467-72. doi: 10.1073/pnas.0405947101. Epub 2004 Sep 21. Proc Natl Acad Sci U S A. 2004. PMID: 15383663 Free PMC article. - CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo.
den Haan JM, Lehar SM, Bevan MJ. den Haan JM, et al. J Exp Med. 2000 Dec 18;192(12):1685-96. doi: 10.1084/jem.192.12.1685. J Exp Med. 2000. PMID: 11120766 Free PMC article. - Immunoproteasomes: structure, function, and antigen presentation.
Ferrington DA, Gregerson DS. Ferrington DA, et al. Prog Mol Biol Transl Sci. 2012;109:75-112. doi: 10.1016/B978-0-12-397863-9.00003-1. Prog Mol Biol Transl Sci. 2012. PMID: 22727420 Free PMC article. Review. - Studying the effect of chloroquine on sporozoite-induced protection and immune responses in Plasmodium berghei malaria.
Bijker EM, Nganou-Makamdop K, van Gemert GJ, Zavala F, Cockburn I, Sauerwein RW. Bijker EM, et al. Malar J. 2015 Mar 26;14:130. doi: 10.1186/s12936-015-0626-2. Malar J. 2015. PMID: 25889324 Free PMC article.
References
- Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403. - PubMed
- York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369. - PubMed
- Rock KL. A new foreign policy: MHC class I molecules police the outside world. Immunol Today. 1996;17:131. - PubMed
- Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I MHC molecules. Science. 1990;24:918. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous