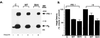Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action - PubMed (original) (raw)
Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action
H Eldar-Finkelman et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The phosphorylation of insulin receptor substrate 1 (IRS-1) on tyrosine residues by the insulin receptor (IR) tyrosine kinase is involved in most of the biological responses of insulin. IRS-1 mediates insulin signaling by recruiting SH2 proteins through its multiple tyrosine phosphorylation sites. The phosphorylation of IRS-1 on serine/threonine residues also occurs in cells; however, the particular protein kinase(s) promoting this type of phosphorylation are unknown. Here we report that glycogen synthase kinase 3 (GSK-3) is capable of phosphorylating IRS-1 and that this modification converts IRS-1 into an inhibitor of IR tyrosine kinase activity in vitro. Expression of wild-type GSK-3 or an "unregulated" mutant of the kinase (S9A) in CHO cells overexpressing IRS-1 and IR, resulted in increased serine phosphorylation levels of IRS-1, suggesting that IRS-1 is a cellular target of GSK-3. Furthermore, insulin-induced tyrosine phosphorylation of IRS-1 and IR was markedly suppressed in cells expressing wild-type or the S9A mutant, indicating that expression of GSK-3 impairs IR tyrosine kinase activity. Taken together, our studies suggest a new role for GSK-3 in attenuating insulin signaling via its phosphorylation of IRS-1 and may provide new insight into mechanisms important in insulin resistance.
Figures
Figure 1
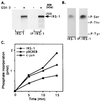
Phosphorylation of IRS-1 by GSK-3. (A) Recombinant IRS-1 (designated as IRS-1) or IRS-1 immunoprecipitated from CHO cells overexpressing IRS-1 (designated as IP-IRS-1) was incubated with recombinant rabbit GSK-3 in the presence of 50 mM Tris (pH 7.3), 10 mM magnesium acetate, 0.01% 2-mercaptoethanol, and 50 μM [γ-32P]ATP (0.25 mCi/ml) in a final volume of 30 μl at 30°C for 15 min. Reactions were stopped by addition of Laemmli sample buffer, subjected to SDS/PAGE, and autoradiographed. (B) Phosphoamino acid composition of each type IRS-1 phosphorylated by GSK-3 was determined as described. The migration positions of phosphorylated tyrosine (P-Tyr), threonine (P-Thr), and serine (P-Ser) are indicated. (C) IRS-1, c-jun, and p9CREB peptide (see Materials and Methods), 10 pmol of each, were phosphorylated by GSK-3 under the conditions described in A for the indicated times. Reactions with IRS-1 or c-jun as the substrates were stopped by addition of Laemmli sample buffer, subjected to SDS/PAGE, and autoradiographed; radioactive bands were cut from the gels and counted for radioactivity. The reaction mixtures containing p9CREB was spotted on phosphocellulose paper and handled as described. Phosphate incorporation into each substrate is presented.
Figure 2
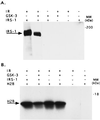
Phosphorylation of IRS-1 by GSK-3 inhibits IR tyrosine kinase activity. (A) Recombinant IRS-1 (0.1 μg) was subjected to sequential phosphorylation reactions, the first involved serine phosphorylation by GSK-3 using unlabeled ATP, and the second involved tyrosine phosphorylation catalyzed by purified IR, again using unlabeled ATP. The reactions were stopped by addition of Laemmli buffer, boiled, and subjected to SDS/PAGE. To detect IR-catalyzed tyrosine phosphorylation of the substrate IRS-1, immunoblot analysis was performed using monoclonal antibodies to phosphotyrosine. Experimental detail are provided in Material and Methods. The composition of the various reactions, including controls are presented in the upper panel of the figure. (B) The same as in A except that 10 μg histone H2B was added together with IR and [γ-32P]ATP. In this instance the phosphorylation of H2B was determined by autoradiography.
Figure 3
Expression of GSK-3 in CHO/IR/IRS-1 cells. Cells were transiently transfected with pCMV4 expression plasmids encoding WT GSK-3β or its S9A mutant as described. GSK-3 expression levels (Upper) were determined by Western blot analysis of cell lysates using polyclonal antibodies to GSK-3β. GSK-3 was immunoprecipitated from cell lysates and kinase activities were assayed in the immunoprecipitate complex using a peptide substrate P-GS1 (Lower). Kinase activities are presented as percent of control (cells transfected with vector alone), and are mean ± SEM from three experiments.
Figure 4
Phosphorylation of IRS-1 by GSK-3 in intact CHO/IR/IRS-1 cells. Transfected cells were metabolically labeled with [32P]orthophosphoric acid for 4 h, and IRS-1 was immunoprecipitated from the cell lysates. Samples were (A) analyzed by gel electrophoresis followed by autoradiography or (B) transferred to polyvinylidene difluoride membrane followed by acid digestion. Phosphoamino acid analysis was performed on the digested samples as described. The migration positions of phosphorylated tyrosine (P-Tyr), threonine (P-Thr), and serine (P-Ser) are indicated.
Figure 5
Insulin-induced tyrosine phosphorylation of IRS-1 and IR in GSK-3-expressing cells. (A) CHO/IR/IRS-1 cells expressing WT GSK-3 or the S9A mutant were starved for 4 hr followed by stimulation with insulin (100 nM) for 15 min. Equal amount of protein from cell lysates were subjected to gel electrophoresis, transferred to polyvinylidene difluoride membrane, and immunoblotted with monoclonal antibodies to phosphotyrosine. Tyrosine phosphorylated IRS-1 and the β subunit of IR are indicated. (B) Image density of tyrosine phosphorylated IRS-1 and the β subunit of IR from A. Results are presented as percent of control cells and are the mean ± SE from three independent experiments.
Similar articles
- Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling.
Liberman Z, Eldar-Finkelman H. Liberman Z, et al. J Biol Chem. 2005 Feb 11;280(6):4422-8. doi: 10.1074/jbc.M410610200. Epub 2004 Nov 30. J Biol Chem. 2005. PMID: 15574412 - Insulin receptor substrate 1 rescues insulin action in CHO cells expressing mutant insulin receptors that lack a juxtamembrane NPXY motif.
Chen D, Van Horn DJ, White MF, Backer JM. Chen D, et al. Mol Cell Biol. 1995 Sep;15(9):4711-7. doi: 10.1128/MCB.15.9.4711. Mol Cell Biol. 1995. PMID: 7651388 Free PMC article. - In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents.
Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. Qiao LY, et al. J Biol Chem. 2002 Jul 19;277(29):26530-9. doi: 10.1074/jbc.M201494200. Epub 2002 May 2. J Biol Chem. 2002. PMID: 12006586 - Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase.
De Fea K, Roth RA. De Fea K, et al. J Biol Chem. 1997 Dec 12;272(50):31400-6. doi: 10.1074/jbc.272.50.31400. J Biol Chem. 1997. PMID: 9395471 - Phosphorylation Codes in IRS-1 and IRS-2 Are Associated with the Activation/Inhibition of Insulin Canonical Signaling Pathways.
Martínez Báez A, Ayala G, Pedroza-Saavedra A, González-Sánchez HM, Chihu Amparan L. Martínez Báez A, et al. Curr Issues Mol Biol. 2024 Jan 9;46(1):634-649. doi: 10.3390/cimb46010041. Curr Issues Mol Biol. 2024. PMID: 38248343 Free PMC article. Review.
Cited by
- GSK-3: Functional Insights from Cell Biology and Animal Models.
Kaidanovich-Beilin O, Woodgett JR. Kaidanovich-Beilin O, et al. Front Mol Neurosci. 2011 Nov 16;4:40. doi: 10.3389/fnmol.2011.00040. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22110425 Free PMC article. - GSK-3 Mouse Models to Study Neuronal Apoptosis and Neurodegeneration.
Gómez-Sintes R, Hernández F, Lucas JJ, Avila J. Gómez-Sintes R, et al. Front Mol Neurosci. 2011 Nov 16;4:45. doi: 10.3389/fnmol.2011.00045. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22110426 Free PMC article. - A novel regulation of IRS1 (insulin receptor substrate-1) expression following short term insulin administration.
Ruiz-Alcaraz AJ, Liu HK, Cuthbertson DJ, McManus EJ, Akhtar S, Lipina C, Morris AD, Petrie JR, Hundal HS, Sutherland C. Ruiz-Alcaraz AJ, et al. Biochem J. 2005 Dec 1;392(Pt 2):345-52. doi: 10.1042/BJ20051194. Biochem J. 2005. PMID: 16128672 Free PMC article. - Type 2 Diabetes Mellitus and Alzheimer's Disease: Shared Molecular Mechanisms and Potential Common Therapeutic Targets.
Hamzé R, Delangre E, Tolu S, Moreau M, Janel N, Bailbé D, Movassat J. Hamzé R, et al. Int J Mol Sci. 2022 Dec 4;23(23):15287. doi: 10.3390/ijms232315287. Int J Mol Sci. 2022. PMID: 36499613 Free PMC article. Review. - Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes.
Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM. Ugi S, et al. Mol Cell Biol. 2004 Oct;24(19):8778-89. doi: 10.1128/MCB.24.19.8778-8789.2004. Mol Cell Biol. 2004. PMID: 15367694 Free PMC article.
References
- Myers M G J, White M F. Annu Rev Pharmacol Toxicol. 1996;36:615–658. - PubMed
- Czech M P. Annu Rev Nutr. 1995;15:441–471. - PubMed
- Saltiel A R. Am J Physiol. 1996;33:E375–E385. - PubMed
- Skolnik E Y, Batzer A, Li N, Lee C H, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. Science. 1993;260:1953–1955. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical