Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia - PubMed (original) (raw)
Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia
Y C Li et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Vitamin D, the major steroid hormone that controls mineral ion homeostasis, exerts its actions through the vitamin D receptor (VDR). The VDR is expressed in many tissues, including several tissues not thought to play a role in mineral metabolism. Studies in kindreds with VDR mutations (vitamin D-dependent rickets type II, VDDR II) have demonstrated hypocalcemia, hyperparathyroidism, rickets, and osteomalacia. Alopecia, which is not a feature of vitamin D deficiency, is seen in some kindreds. We have generated a mouse model of VDDR II by targeted ablation of the second zinc finger of the VDR DNA-binding domain. Despite known expression of the VDR in fetal life, homozygous mice are phenotypically normal at birth and demonstrate normal survival at least until 6 months. They become hypocalcemic at 21 days of age, at which time their parathyroid hormone (PTH) levels begin to rise. Hyperparathyroidism is accompanied by an increase in the size of the parathyroid gland as well as an increase in PTH mRNA levels. Rickets and osteomalacia are seen by day 35; however, as early as day 15, there is an expansion in the zone of hypertrophic chondrocytes in the growth plate. In contrast to animals made vitamin D deficient by dietary means, and like some patients with VDDR II, these mice develop progressive alopecia from the age of 4 weeks.
Figures
Figure 1
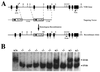
Targeting strategy for VDR ablation. (A) Construction of targeting vector for VDR ablation. A schematic representation of the VDR gene is shown, based on the structure of the human gene and characterization of the sequences from exon 3 to exon 9 of the mouse gene. The exons are numbered and indicated by solid boxes. A partial restriction map is shown for the following enzymes: R, _Eco_RI; X, _Xba_I; S, _Sac_I. The _Xba_I site indicated by the asterisk was derived from the phage arm. A 5-kb _Xba_I fragment 5′ to exon 3 and a 3.5-kb _Xba_I fragment 3′ to exon 3 of the mouse VDR gene were used as the targeting sequences. The _Sac_I-_Xba_I fragment used as a probe for identifying homologous recombinants is indicated. (B) Genomic Southern analysis of tail DNA derived from offspring of heterozygous matings. The DNA was digested with _Eco_RI and hybridized with the external probe as indicated in A. The wild-type allele generates a 22-kb fragment, and the mutant allele resulting from homologous recombination produces a 15-kb fragment. Wild-type mice are indicated by +/+; heterozygous, by +/−; and homozygous receptor ablated mice, by −/−.
Figure 2
Growth and serum chemistries of the VDR ablated mice. (A) Male mice of all three genotypes were weighed at weekly intervals from 2 to 13 weeks of age. The wild-type (squares) and heterozygous (circles) mice grow at a similar rate; however, the homozygous ablated (diamonds) mice gain weight more slowly after weaning (at 21 days of age). All values are based on three to five animals, and errors represent the SEM. Absence of error bars indicates points where the SEM is less than 0.6. The shape of the female weight curve is similar. (B) Ionized calcium measurements (in mmol/L, corrected for pH) were performed at weekly intervals from 2 to 13 weeks of age. Day 14 samples were obtained by cardiac puncture under isoflurane anesthesia. All other samples were obtained by tail nicking. The wild-type (squares) and heterozygous (circles) mice have similar levels, whereas the homozygous mice (diamonds) become hypocalcemic after weaning. All values represent the mean and SEM based on measurements in three to five animals. Absence of error bars indicates points where the SEM is less than 0.01. There was no sex difference in ionized calcium values. (C) Immunoreactive PTH values were measured using a rat PTH assay. iPTH values in the wild-type (squares) and heterozygous mice (data not shown) were less than 18 pg/ml at all stages. iPTH levels in the vitamin D receptor ablated mice (diamonds) were indistinguishable from those of their heterozygous and wild-type littermates at 15 and 19 days of age but by 21 days began to rise. All values are based on the mean and SEM of sera from three mice of the same genotype.
Figure 3
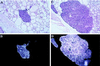
In situ hybridization of parathyroid glands from VDR ablated mice with a rat PTH probe. (A) 25× magnification of hematoxylin and eosin counter-stained bright-field section of a parathyroid gland from 70-day-old +/+ mouse hybridized in situ for PTH mRNA. (B) Dark-field exposure of A. (C) 25× magnification of hematoxylin and eosin counter-stained bright-field section of a parathyroid gland from a 70-day-old −/− littermate hybridized in situ for PTH mRNA. (D) Dark-field exposure of C. No signal was observed using the sense probe.
Figure 4
Contact radiography and histology of the tibia of VDR ablated mice. (A) Contact x-ray of the tibia of a 35-day-old control mouse. (B) Contact x-ray of the tibia of a 35-day-old −/− littermate. (C) von Kossa stain of a nondemineralized section through the tibia of the 35-day-old control mouse. (D) von Kossa stain of a nondemineralized section through the tibia of a 35-day-old −/− littermate. (E) Toluidine blue section through the growth plate of the 35-day-old control mouse. (F) Toluidine blue section through the growth plate of the 35-day-old −/− littermate. (G) Toluidine blue section through the growth plate of a 15-day-old control mouse. (H) Toluidine blue section through the growth plate of a 15-day-old −/− littermate.
Figure 5
Appearance of VDR ablated mice at 3.5 months of age. The genotypes of the mice, from left to right, are wild type, heterozygous, and homozygous ablated.
Similar articles
- Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice.
Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Li YC, et al. Endocrinology. 1998 Oct;139(10):4391-6. doi: 10.1210/endo.139.10.6262. Endocrinology. 1998. PMID: 9751523 - Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D.
Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, Möller G, Adamski J, Balling R. Erben RG, et al. Mol Endocrinol. 2002 Jul;16(7):1524-37. doi: 10.1210/mend.16.7.0866. Mol Endocrinol. 2002. PMID: 12089348 - Presence of a deletion mutation (c.716delA) in the ligand binding domain of the vitamin D receptor in an Indian patient with vitamin D-dependent rickets type II.
Kanakamani J, Tomar N, Kaushal E, Tandon N, Goswami R. Kanakamani J, et al. Calcif Tissue Int. 2010 Jan;86(1):33-41. doi: 10.1007/s00223-009-9310-2. Epub 2009 Nov 17. Calcif Tissue Int. 2010. PMID: 19921089 - Mechanism of vitamin D receptor action.
Demay MB. Demay MB. Ann N Y Acad Sci. 2006 Apr;1068:204-13. doi: 10.1196/annals.1346.026. Ann N Y Acad Sci. 2006. PMID: 16831920 Review. - In vivo function of VDR in gene expression-VDR knock-out mice.
Kato S, Takeyama K, Kitanaka S, Murayama A, Sekine K, Yoshizawa T. Kato S, et al. J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1-6):247-51. doi: 10.1016/s0960-0760(99)00042-4. J Steroid Biochem Mol Biol. 1999. PMID: 10418998 Review.
Cited by
- 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages.
Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, Yoon D, Kong J, Thadhani R, Li YC. Chen Y, et al. J Immunol. 2013 Apr 1;190(7):3687-95. doi: 10.4049/jimmunol.1203273. Epub 2013 Feb 22. J Immunol. 2013. PMID: 23436936 Free PMC article. - Pleiotropic Activities of Vitamin D Receptors - Adequate Activation for Multiple Health Outcomes.
Ryan JW, Anderson PH, Morris HA. Ryan JW, et al. Clin Biochem Rev. 2015 May;36(2):53-61. Clin Biochem Rev. 2015. PMID: 26224895 Free PMC article. Review. - Nutritionally-induced catch-up growth.
Gat-Yablonski G, Phillip M. Gat-Yablonski G, et al. Nutrients. 2015 Jan 14;7(1):517-51. doi: 10.3390/nu7010517. Nutrients. 2015. PMID: 25594438 Free PMC article. Review. - Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway.
Beildeck ME, Gelmann EP, Byers SW. Beildeck ME, et al. Exp Cell Res. 2010 Jul 1;316(11):1763-72. doi: 10.1016/j.yexcr.2010.02.001. Epub 2010 Feb 6. Exp Cell Res. 2010. PMID: 20138864 Free PMC article. Review. - Endogenous retinoids in the hair follicle and sebaceous gland.
Everts HB. Everts HB. Biochim Biophys Acta. 2012 Jan;1821(1):222-9. doi: 10.1016/j.bbalip.2011.08.017. Epub 2011 Sep 3. Biochim Biophys Acta. 2012. PMID: 21914489 Free PMC article. Review.
References
- Li Y, Bergwitz C, Jüppner H, Demay M. Endocrinology. 1997;138:2347–2353. - PubMed
- Johnson J A, Grande J P, Roche P C, Kumar R. J Bone Miner Res. 1996;11:56–61. - PubMed
- Elaroussi M A, Uhland-Smith A, Hellwig W, DeLuca H F. Biochim Biophys Acta. 1994;1192:1–6. - PubMed
- Tuan R S, Suyama E. J Nutr. 1996;126:1308S–1316S. - PubMed
- Stumpf W E, Sar M, Reid F A, Tanaka Y, DeLuca H F. Science. 1979;206:1188–1190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases