A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA - PubMed (original) (raw)
A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA
M L Grimes et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The topology of signal transduction is particularly important for neurons. Neurotrophic factors such as nerve growth factor (NGF) interact with receptors at distal axons and a signal is transduced by retrograde transport to the cell body to ensure survival of the neuron. We have discovered an organelle that may account for the retrograde transport of the neurotrophin signal. This organelle is derived from endocytosis of the receptor tyrosine kinase for NGF, TrkA. In vitro reactions containing semi-intact PC12 cells and ATP were used to enhance recovery of a novel organelle: small vesicles containing internalized NGF bound to activated TrkA. These vesicles were distinct from clathrin coated vesicles, uncoated primary endocytic vesicles, and synaptic vesicles, and resembled transport vesicles in their sedimentation velocity. They contained 10% of the total bound NGF and almost one-third of the total tyrosine phosphorylated TrkA. These small vesicles are compelling candidates for the organelles through which the neurotrophin signal is conveyed down the axon.
Figures
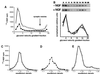
Figure 3
(A) TrkA was crosslinked to NGF in small endocytic vesicles. Cells were bound to 125I-NGF, washed, warmed 10 min, chilled and analyzed after an in vitro reaction with ATP. The membrane permeable crosslinking reagent disuccinimidyl suberate was added (2 mM, 4°C, 30 min) to the permeabilized cell suspension before fractionation of membranes. One-fifth of the cell ghost membranes (1,000 × g pellet, P1), one-half of the 8,000 × g pellet (P2) one-half of the 100,000 × g pellet (P3), and one-tenth of the 100,000 × g supernatant (S3) were immunoprecipitated from samples with anti-TrkA (1088) (20, 42) and analyzed by SDS/PAGE and autoradiography (22 day exposure). The position of molecular weight markers (kDa) is indicated. (B) TrkA is still activated in intracellular organelles after in vitro reactions. Untreated PC12 cells or PC12 cells bound to NGF (1 nM, 4°C) were warmed 10 min and fractionated and immunoprecipitated as in A after in vitro incubation with ATP. The presence of TrkA was assessed by immunoprecipitation, followed by Western blotting, with anti-TrkA and anti-phosphotyrosine (indicated). RTA (a gift of D. Clary, ref. 72) was used for immunoprecipitations. Western blots were probed with RTA followed by horseradish peroxidase-conjugated anti-rabbit IgG and ECL (Left, 1 min exposure). Two proteins were identified, gp140TrkA and gp110TrkA (indicated). The latter is a precursor to gp140TrkA (41, 42), and remained mostly with the cell ghosts after in vitro reactions with ATP. TrkA immunoprecipitates were also probed with anti-phosphotyrosine (4G10, a gift of S. Robbins and M. Bishop, University of California, San Francisco) followed by 125I-goat anti-mouse IgG (Right, 34 day exposure). Mature 140-kDa TrkA was tyrosine phosphorylated, while the 110-kDa immature glycosylated TrkA was not. Equal amounts of cells were used to compare conditions. The top and bottom edges of the panels mark the position of the 200- and 97.4-kDa molecular weight markers, respectively. (C–E) Quantification of TrkA and tyrosine phosphorylated TrkA in intracellular organelles. Data for gp140TrkA (C) and gp110TrkA (D) from 4 experiments as in B (Left) were quantified by densitometry and plotted as a percent of the total in all cell fractions with error bars showing standard deviations. The conditions and fractions are labeled at the left. Data for tyrosine phosphorylated TrkA (E) from four experiments as in (B Right) were quantified by phosphorimaging or densitometry and plotted in reference to total tyrosine phosphorylated TrkA following NGF treatment with error bars as in C. (F) Sodium orthovanadate was added to in vitro incubations with ATP. Untreated cells (Left) and NGF-treated cells (Right) were permeabilized and incubated in vitro with ATP in the presence of 1 mM sodium orthovanadate. TrkA was immunoprecipitated as in A with anti-TrkA (1,088) (20, 42). In the P3 fraction, PLC-γ, then TrkA was immunoprecipitated from the P3 fractions (indicated by IP). Proteins were Western blotted with antiphosphotyrosine followed by horseradish peroxidase-conjugated antimouse and detected using ECL (20 sec exposure). Equal amounts of cells were used to compare conditions. The top and bottom edges of the panels mark the position of the 200- and 97.4-kDa molecular weight markers, respectively.
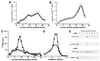
Figure 1
(A and B) Sucrose velocity sedimentation resolved different classes of vesicles containing 125I-NGF. Cells bound to 1 nM 125I-NGF at 4°C were washed, warmed 10 min in vivo, chilled, permeabilized, then subjected to in vitro reactions by warming for 15 min either in the presence of (A) an ATP depleting system (−ATP, •) or (B) an ATP regenerating system (+ATP, □). Released vesicles were separated from cell ghosts, concentrated by 100,000 × g centrifugation, and applied to sucrose velocity gradients. Gradient fractions were collected from the bottom of the tube, so that the largest organelles were in the lowest numbered fractions. Shown is the amount of 125I-NGF in gradient fractions expressed as a percent of the total from all cell fractions (20). Error bars indicate the SD of measurements from four (A) or six (B) experiments. (C and D) Large vesicles containing internalized NGF are clathrin-coated primary endocytic vesicles. Vesicles that emerged from permeabilized cells were fractionated by centrifuging 8,000 × g for 35 min (P2), either directly after warming 10 min in vivo, (•), or after warming and an in vitro reaction with ATP (+ATP, □). Pellets were resuspended and applied to sucrose velocity (C) and equilibrium (D) gradients. Representative experiments are plotted on the same _y_-axis scale. (E) Clathrin and α-adaptins were localized in the P2 in velocity gradient fractions 6–11 with or without in vitro reactions under all conditions. Shown is a Western blot of positive gradient fractions from in vitro reactions with an ATP-depleting system (−ATP) and ATP regenerating system (+ATP). Monoclonal antibodies TD.1 (70) and AP.6 (71) (a gift of F. Brodsky, University of California, San Francisco) detected clathrin (180 kDa) and two α-adaptins (a doublet centered around 100 kDa), respectively, using enhanced chemiluminescence (Amersham) for detection (1 hr exposure).
Figure 2
(A and B) Synaptic vesicles did not contain NGF. (A) Cells bound to 125I-NGF were washed, warmed 10 min in vivo, chilled, washed, then permeabilized. Cells were fractionated by successive 1,000 × g (P1), 8,000 × g (P2), and 100,000 × g (P3) centrifugations before or after in vitro reactions with ATP. Vesicles in the P3 without an in vitro reaction, (•) or after an in vitro reaction with ATP (+ATP, □) were applied to 5–25% glycerol velocity gradients and centrifuged 200,000 × g for 1 hr. (B) Synaptophysin in cell fractions was quantified by Western blotting. After incubating with 1 nM NGF or the vehicle alone at 4°C for 1 hr, PC12 cells were warmed 10 min at 37°C, chilled, washed, and permeabilized without in vitro reactions. Glycerol velocity gradient fractions (as in A except that fractions were pooled in pairs) were analyzed by SDS/PAGE, proteins were transferred to nitrocellulose and probed with anti-synaptophysin (SY38), followed by goat anti-mouse and 125I-protein A. Autoradiograms of Western blots (above, 3 day exposure) and PhosphorImager quantification (below) show the amount of synaptophysin present in fractions in the absence of NGF (−NGF, •) or its presence (+NGF, □). The distribution of rab3A (a gift of L. Elferink and R. Scheller, Stanford University, Stanford; not shown) and synaptophysin identified synaptic vesicles in a peak at fractions 14–16. (C–E) In vitro reactions capture intermediates at different stages of endocytosis. Equilibrium density of 125I-NGF-containing vesicles in the 100,000 × g P3 (plotted on the same _y_-axis scale) after 2 min internalization in vivo plus a 15 min in vitro reaction with ATP (C, +ATP, ▵), or after 10 min internalization in vivo, plus a 15 min in vitro reaction without ATP (D, −ATP, •), or after 10 min internalization in vivo, plus a 15 min in vitro reaction with ATP (E, +ATP, □).
Similar articles
- Rapid retrograde tyrosine phosphorylation of trkA and other proteins in rat sympathetic neurons in compartmented cultures.
Senger DL, Campenot RB. Senger DL, et al. J Cell Biol. 1997 Jul 28;138(2):411-21. doi: 10.1083/jcb.138.2.411. J Cell Biol. 1997. PMID: 9230082 Free PMC article. - Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes.
Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Grimes ML, et al. J Neurosci. 1996 Dec 15;16(24):7950-64. doi: 10.1523/JNEUROSCI.16-24-07950.1996. J Neurosci. 1996. PMID: 8987823 Free PMC article. - An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons.
Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. Riccio A, et al. Science. 1997 Aug 22;277(5329):1097-100. doi: 10.1126/science.277.5329.1097. Science. 1997. PMID: 9262478 - Studying signal transduction in neuronal cells: the Trk/NGF system.
Kaplan DR. Kaplan DR. Prog Brain Res. 1998;117:35-46. doi: 10.1016/s0079-6123(08)64005-4. Prog Brain Res. 1998. PMID: 9932398 Review. No abstract available. - NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.
Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. Bradshaw RA, et al. Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
Cited by
- Neurotrophin mimetics and tropomyosin kinase receptors: a futuristic pharmacological tool for Parkinson's.
J JB, Palathoti N, Dhanasekaran M, Sivasamy R, Ponnusankar S, Dhanabal SP, Sankar V, Justin A. J JB, et al. Neurol Sci. 2023 Jul;44(7):2265-2275. doi: 10.1007/s10072-023-06684-1. Epub 2023 Mar 4. Neurol Sci. 2023. PMID: 36870001 Review. - γ-Enolase enhances Trk endosomal trafficking and promotes neurite outgrowth in differentiated SH-SY5Y cells.
Pišlar A, Kos J. Pišlar A, et al. Cell Commun Signal. 2021 Dec 11;19(1):118. doi: 10.1186/s12964-021-00784-1. Cell Commun Signal. 2021. PMID: 34895236 Free PMC article. - NGF Signaling in Endosomes.
Yamashita N. Yamashita N. Adv Exp Med Biol. 2021;1331:19-29. doi: 10.1007/978-3-030-74046-7_3. Adv Exp Med Biol. 2021. PMID: 34453290 - Nerve Growth Factor as an Ocular Therapy: Applications, Challenges, and Future Directions.
Kanu LN, Ciolino JB. Kanu LN, et al. Semin Ophthalmol. 2021 May 19;36(4):224-231. doi: 10.1080/08820538.2021.1890793. Epub 2021 Feb 27. Semin Ophthalmol. 2021. PMID: 33641595 Free PMC article. - Mechanisms for Regulating and Organizing Receptor Signaling by Endocytosis.
von Zastrow M, Sorkin A. von Zastrow M, et al. Annu Rev Biochem. 2021 Jun 20;90:709-737. doi: 10.1146/annurev-biochem-081820-092427. Epub 2021 Feb 19. Annu Rev Biochem. 2021. PMID: 33606955 Free PMC article. Review.
References
- Misko T P, Radeke M J, Shooter E M. J Exp Biol. 1987;132:177–190. - PubMed
- Halegoua S, Armstrong R C, Kremer N E. Curr Top Microbiol Immunol. 1991;165:119–170. - PubMed
- Grimes M, Zhou J, Li Y, Holtzman D, Mobley W C. Semin Neurosci. 1993;5:239–247.
- Baass P C, Di Guglielmo B M, Authier F, Posner B I, Bergeron J J. Trends Cell Biol. 1995;5:465–475. - PubMed
- Tooze J, Hollinshead M, Fuller S D, Tooze S A, Huttner W B. Eur J Cell Biol. 1989;49:259–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources