VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity - PubMed (original) (raw)
VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity
N Takahashi et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The brain vesicular monoamine transporter (VMAT2) pumps monoamine neurotransmitters and Parkinsonism-inducing dopamine neurotoxins such as 1-methyl-4-phenyl-phenypyridinium (MPP+) from neuronal cytoplasm into synaptic vesicles, from which amphetamines cause their release. Amphetamines and MPP+ each also act at nonvesicular sites, providing current uncertainties about the contributions of vesicular actions to their in vivo effects. To assess vesicular contributions to amphetamine-induced locomotion, amphetamine-induced reward, and sequestration and resistance to dopaminergic neurotoxins, we have constructed transgenic VMAT2 knockout mice. Heterozygous VMAT2 knockouts are viable into adult life and display VMAT2 levels one-half that of wild-type values, accompanied by smaller changes in monoaminergic markers, heart rate, and blood pressure. Weight gain, fertility, habituation, passive avoidance, and locomotor activities are similar to wild-type littermates. In these heterozygotes, amphetamine produces enhanced locomotion but diminished behavioral reward, as measured by conditioned place preference. Administration of the MPP+ precursor N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to heterozygotes produces more than twice the dopamine cell losses found in wild-type mice. These mice provide novel information about the contributions of synaptic vesicular actions of monoaminergic drugs and neurotoxins and suggest that intact synaptic vesicle function may contribute more to amphetamine-conditioned reward than to amphetamine-induced locomotion.
Figures
Figure 1
Construction and characterization of VMAT2 knockout mice. (A) Representation of the wild-type and predicted knockout genomic sequences, with prominent restriction endonuclease sites, neomycin-resistance sequences, and sequences recognized by the pVMAT2ko5′ and pVMAT2ko3′ hybridization probes as indicated. Construction of pBS_neo_V2KO targeting vector indicating prominent restriction endonuclease cleavage sites and sites for the neomycin resistance (Neo) and of the thymidine kinase (TK) sequences. (B) Southern blot analyses of hybridization of radiolabeled pVMAT2ko5′ (Left) and pVMAT2ko3′ (Right) hybridization probes with genomic DNA extracted from the tails of wild-type (+/+), heterozygote (+/−), and homozygote (−/−) mice. Longer fragments result from homologous recombination replacing VMAT2 sequences including exons I–III with the neomycin-resistance cassette. (C) Scatchard analyses of saturation radioligand binding data using [3H]dihydrotetrabenazine and striatal membranes prepared from 6-week-old wild-type and heterozygous knockout mice and from whole-brain membranes prepared from postnatal day 1 wild-type, heterozygote, and homozygote knockout animals (Inset). (D) Kaplan–Meier survival plots of wild-type, heterozygous, and homozygous knockout mice. Approximately 15 mice of each genotype were followed for 2 months. (E) Weight gains of wild-type (+/+) and heterozygote (+/−) knockout mice.
Figure 2
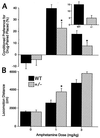
Amphetamine effects in wild-type and heterozygous VMAT2 knockout mice. (A) Conditioned place preference induced by pairing indicated amphetamine doses with the initially nonpreferred side of the apparatus or pairing 1 mg/kg amphetamine with the initially preferred side of the apparatus (Inset) in heterozygote VMAT2 knockout mice or wild-type littermates. ∗, P < 0.05, comparing heterozygotes with wild-type littermates. (B) Locomotion induced by indicated amphetamine doses in heterozygote VMAT2 knockout mice or wild-type littermates. ∗, P < 0.05, comparing heterozygotes with wild-type littermates.
Figure 3
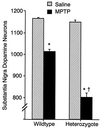
MPTP toxicity in wild-type and heterozygous VMAT2 knockout mice. Numbers of substantia nigra, pars compacta TH immunoreactive dopaminergic neurons in wild-type and heterozygote VMAT2 knockout mice treated with saline or MPTP (24). These doses produced similar brain levels of MPP+ in mice of both genotypes in preliminary studies. ∗, P < 0.05, compared with saline injection. †, MPTP effect significantly different from its effect in wild-type mice.
Similar articles
- Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice.
Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Gainetdinov RR, et al. J Neurochem. 1998 May;70(5):1973-8. doi: 10.1046/j.1471-4159.1998.70051973.x. J Neurochem. 1998. PMID: 9572281 - Presynaptic control of striatal dopamine neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice.
Patel J, Mooslehner KA, Chan PM, Emson PC, Stamford JA. Patel J, et al. J Neurochem. 2003 May;85(4):898-910. doi: 10.1046/j.1471-4159.2003.01732.x. J Neurochem. 2003. PMID: 12716422 - The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins.
Uhl GR, Li S, Takahashi N, Itokawa K, Lin Z, Hazama M, Sora I. Uhl GR, et al. FASEB J. 2000 Dec;14(15):2459-65. doi: 10.1096/fj.00-0205rev. FASEB J. 2000. PMID: 11099463 Review. - Impact of psychostimulants on vesicular monoamine transporter function.
Fleckenstein AE, Hanson GR. Fleckenstein AE, et al. Eur J Pharmacol. 2003 Oct 31;479(1-3):283-9. doi: 10.1016/j.ejphar.2003.08.077. Eur J Pharmacol. 2003. PMID: 14612158 Review.
Cited by
- Effect of altered production and storage of dopamine on development and behavior in C. elegans.
Lee I, Knickerbocker AC, Depew CR, Martin EL, Dicent J, Miller GW, Bucher ML. Lee I, et al. Front Toxicol. 2024 Aug 16;6:1374866. doi: 10.3389/ftox.2024.1374866. eCollection 2024. Front Toxicol. 2024. PMID: 39219718 Free PMC article. - Synthesis and evaluation of a series of tropane analogues as novel vesicular monoamine transporter-2 ligands.
Zheng G, Dwoskin LP, Deaciuc AG, Crooks PA. Zheng G, et al. Bioorg Med Chem Lett. 2005 Oct 15;15(20):4463-6. doi: 10.1016/j.bmcl.2005.07.032. Bioorg Med Chem Lett. 2005. PMID: 16112864 Free PMC article. - Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size.
Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Pothos EN, et al. J Neurosci. 2000 Oct 1;20(19):7297-306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. J Neurosci. 2000. PMID: 11007887 Free PMC article. - VMAT2 and dopamine neuron loss in a primate model of Parkinson's disease.
Chen MK, Kuwabara H, Zhou Y, Adams RJ, Brasić JR, McGlothan JL, Verina T, Burton NC, Alexander M, Kumar A, Wong DF, Guilarte TR. Chen MK, et al. J Neurochem. 2008 Apr;105(1):78-90. doi: 10.1111/j.1471-4159.2007.05108.x. Epub 2007 Nov 5. J Neurochem. 2008. PMID: 17988241 Free PMC article. - Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons.
Guillot TS, Miller GW. Guillot TS, et al. Mol Neurobiol. 2009 Apr;39(2):149-70. doi: 10.1007/s12035-009-8059-y. Epub 2009 Mar 4. Mol Neurobiol. 2009. PMID: 19259829 Review.
References
- Johnson R G. Physiol Rev. 1988;68:232–307. - PubMed
- Henry J-P, Botton D, Sagne C, Isambert M-F, Desnos C, Blanchard V, Raisman-Vozari R, Krejci E, Massoulie J, Gasnier B. J Exp Biol. 1994;196:251–262. - PubMed
- Liu Y, Peter D, Roghani A, Schuldiner S, Privé G G, Eisenberg D, Brecha N, Edwards R H. Cell. 1992;70:539–551. - PubMed
- National Institute of Justice. Annual Report on Adult and Juvenile Arrestees. Rockville, MD: Natl. Criminal Justice Ref. Serv.; 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases