Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis - PubMed (original) (raw)
Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis
M Majdan et al. J Neurosci. 1997.
Abstract
We have asked whether p75(NTR) may play a role in neuronal apoptosis by producing transgenic mice that express the p75(NTR) intracellular domain within peripheral and central neurons. These animals showed profound reductions in numbers of sympathetic and peripheral sensory neurons as well as cell loss in the neocortex, where there is normally little or no p75(NTR) expression. Developmental loss of facial motor neurons was not observed, but induced expression of the p75(NTR) intracellular domain within adult animals led to increased motor neuron death after axotomy. Biochemical analyses suggest that these effects were not attributable to a p75(NTR)-dependent reduction in trk activation but instead indicate that the p75(NTR) intracellular domain may act as a constitutive activator of signaling cascades that regulate apoptosis in both peripheral and central neurons.
Figures
Fig. 7.
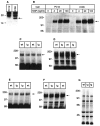
Expression of the p75NTR-ICD does not affect trk receptor levels or autophosphorylation. A, B, Stable PC12 cells lines created to overexpress the p75NTR-ICD were analyzed for trkA autophosphorylation. A, p75NTR-ICD was detected by immunoblot in these lines, using αp1 directed against the p75NTRintracellular domain. B, Effects of the p75NTR-ICD on NGF-induced trkA autophosphorylation were determined in stable PC12 cell lines subjected to a 5 min treatment with either vehicle or with 4, 20, or 100 ng/ml NGF. TrkA was immunoprecipitated with anti-pan-trk 203 and subsequently was analyzed for phosphotyrosine content by immunoblot, as described in Materials and Methods. C–F, Trk receptor levels and endogenous trk tyrosine phosphorylation are similar in cortices (C, D) of neonatal animals of line 4173 and their control littermates. Cortical lysates were immunoprecipitated with pan-trk antibody 203 and analyzed on immunoblots with anti-phosphotyrosine antibody 4G10 (C). The same blots subsequently were reprobed with trkBout, which is specific for trkB (D). No difference in trk activation or levels was observed between control and transgenic animals. E, F, Trk receptor levels and endogenous trk tyrosine phosphorylation are similar in the cortex of adult animals of line 4173 and their control littermates. E, Total trk receptors immunoprecipitated from adult cortex were analyzed for phosphotyrosine content by 4G10 immunoblot. F, Anti-pan-trk immunoprecipitates from adult animals were immunoblotted to detect total trk receptor levels, using anti-pan-trk 203. G, Lysates of neonatal cortex from line 4173 and control animals were analyzed for levels of phosphotyrosine-containing proteins by immunoblot with 4G10. The arrow in _A_indicates the p75NTR-ICD; the _arrows_in B–F indicate trk receptors. Molecular weight standards are indicated on the left side of each panel.
Fig. 1.
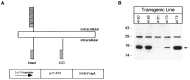
Expression of the Tα1:ICD in the brains of transgenic mice. A, A Tα1 minigene cloning cassette was constructed in which the 1.1 kb Tα1 α-tubulin promoter was separated from an SV40 intron and polyadenylation sites by a cDNA encoding amino acids 276–425 of the rat p75NTR. The resultant minigene was purified free from vector sequence and microinjected to produce founder transgenic animals at the Canadian NeuroScience Network Transgenic Core Facility (McGill University).B, Expression of the p75NTR-ICD in the brains of various lines of Tα1:p75NTR-ICD mice detected by immunoblot. The position of the p75NTR-ICD is indicated by an arrow(right), and molecular weight standards are indicated (left).
Fig. 2.
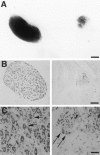
Expression of the p75NTR-ICD in developing neurons leads to loss of sympathetic neurons.A, Sympathetic superior cervical ganglion (SCG) from a postnatal day 1 Tα1:nlacZ control mouse (left) and from its Tα1:ICD × Tα1:nlacZ littermate (line 4173,right) were stained with X-gal to visualize Tα1:nlacZ-expressing neurons. Scale bar, 167 μm. B, Photomicrograph of SCG sections from an adult control (left) or from a line 4173 Tα1:ICD mouse (right) stained with cresyl violet. Scale bar, 84 μm.C, Higher magnification of sections of sympathetic cervical ganglia from a wild-type (left) or from a 4173 Tα1:ICD mouse (right). Arrows in_C_ indicate sympathetic neurons. Scale bar, 21 μm.
Fig. 3.
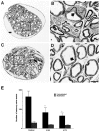
Peripheral sensory neurons are lost in p75NTR-ICD transgenic mice. A, Whole-mount E13.5 embryos derived from a Tα1:ICD × Tα1:nLacZ cross were stained with X-gal. Arrows indicate DRG within a control embryo (left) and within an embryo carrying the Tα1:ICD transgene (right). This example shows a severely affected animal. Scale bar, 400 μm.B, Counts of neuronal profiles in L3, L4, and L5 DRG of adult wild-type or line 4173 Tα1:ICD animals revealed a significant loss of sensory neurons (*p < 0.05).
Fig. 4.
Expression of the p75NTR-ICD leads to loss of unmyelinated sensory axons of the dorsal cutaneous nerve. A–D, Cross sections of the dorsal cutaneous nerve of adult control mice (A, B) and adult Tα1:ICD mice of line 4163 (C, D), as visualized by electron microscopy. B, D, Higher magnifications of the boxes outlined in A and_C_, respectively. Note the selective loss of the smaller fiber unmyelinated axons (thin arrows) relative to the large fiber myelinated sensory fibers (thick arrows). Scale bar, 6 μm. E, Quantitation of axonal loss demonstrates a selective loss of small unmyelinated sensory fibers in the DCN of Tα1:ICD mice of lines 4163 and 4173 relative to controls (*p < 0.05). The myelinated population measured in this analysis includes both the small- and large-caliber myelinated sensory axons.
Fig. 5.
Neuronal expression of the p75NTR-ICD leads to the loss of neurons within the neocortex. Shown are photomicrographs of Nissl-stained coronal sections of the neocortex of control and transgenic p75NTR-ICD animals. Right and_far left_ photographic panels are from control animals, whereas the three inner panels are all from transgenic animals of the 4173 line. The area examined is indicated in the schematic drawings shown at left, with the top panels representing the rostral level of the neocortex and the bottom panels representing the caudal level. Each vertically aligned pair of photomicrographs is derived from the same animal. Brackets in the far left panel indicate approximate boundaries of the different cortical layers. Scale bar, 75 μm. Neuronal counts reveal a highly significant loss of cortical neurons in the p75NTR-ICD (p < 0.001; see Results).
Fig. 6.
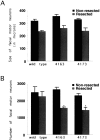
Inducible expression of the p75NTR-ICD results in loss of mature adult facial motor neurons after nerve injury. Coronal sections of facial nuclei were prepared from adult animals subjected to a unilateral facial nerve lesion 7 d earlier. Sections were stained with cresyl violet, and neuronal counts were performed on every fifth section, as described in Materials and Methods. Neither the size (A; in μm2) nor the number of facial motor neurons (B) is affected significantly by developmental expression of the p75NTR-ICD in uninjured neurons, but lesion of the facial nerve results in loss of facial motor neurons in ICD-expressing animals (*p< 0.05).
Fig. 8.
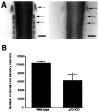
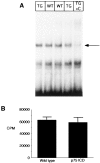
Levels of NF-kB and jun kinase activity are not altered in brain lysates of embryonic transgenic animals.A, Nuclear extracts from E16 brain lysates were analyzed for NF-kB activity by electrophoretic mobility shift, using an end-labeled 32 bp fragment derived from an HIV-LTR as probe. In_lane 5_ (TG + C), 50 ng of an unlabeled NF-kB element was included with the lane 4 sample to determine specific NF-kB binding complexes (indicated by_arrow_). Shifted patterns are similar in wild-type (lanes 2 and 3) and in transgenic line 4173 (lanes 1 and 4) brains. This is typical of four similar experiments. B, Brain lysates from a litter of E16 animals were analyzed for jnk activity, as described in Materials and Methods. Results shown represent mean of assays of individual complete litter (n = 5 wild-type and 4 for Tα1:ICD mice) and represent one of three similar experiments.
Similar articles
- p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors.
Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD. Aloyz RS, et al. J Cell Biol. 1998 Dec 14;143(6):1691-703. doi: 10.1083/jcb.143.6.1691. J Cell Biol. 1998. PMID: 9852160 Free PMC article. - The p75 neurotrophin receptor and neuronal apoptosis.
Barrett GL. Barrett GL. Prog Neurobiol. 2000 Jun;61(2):205-29. doi: 10.1016/s0301-0082(99)00056-8. Prog Neurobiol. 2000. PMID: 10704998 Review. - Neurotrophin signaling through the p75 receptor is deficient in traf6-/- mice.
Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Yeiser EC, et al. J Neurosci. 2004 Nov 17;24(46):10521-9. doi: 10.1523/JNEUROSCI.1390-04.2004. J Neurosci. 2004. PMID: 15548667 Free PMC article. - Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers.
Vilar M, Charalampopoulos I, Kenchappa RS, Simi A, Karaca E, Reversi A, Choi S, Bothwell M, Mingarro I, Friedman WJ, Schiavo G, Bastiaens PI, Verveer PJ, Carter BD, Ibáñez CF. Vilar M, et al. Neuron. 2009 Apr 16;62(1):72-83. doi: 10.1016/j.neuron.2009.02.020. Neuron. 2009. PMID: 19376068 Free PMC article. - Expression and function of Xenopus laevis p75(NTR) suggest evolution of developmental regulatory mechanisms.
Hutson LD, Bothwell M. Hutson LD, et al. J Neurobiol. 2001 Nov 5;49(2):79-98. doi: 10.1002/neu.1067. J Neurobiol. 2001. PMID: 11598917 Review.
Cited by
- Suppression of p75 neurotrophin receptor surface expression with intrabodies influences Bcl-xL mRNA expression and neurite outgrowth in PC12 cells.
Zhang C, Helmsing S, Zagrebelsky M, Schirrmann T, Marschall AL, Schüngel M, Korte M, Hust M, Dübel S. Zhang C, et al. PLoS One. 2012;7(1):e30684. doi: 10.1371/journal.pone.0030684. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22292018 Free PMC article. - PolyADP-ribosylation is involved in neurotrophic activity.
Visochek L, Steingart RA, Vulih-Shultzman I, Klein R, Priel E, Gozes I, Cohen-Armon M. Visochek L, et al. J Neurosci. 2005 Aug 10;25(32):7420-8. doi: 10.1523/JNEUROSCI.0333-05.2005. J Neurosci. 2005. PMID: 16093393 Free PMC article. - Siva-1 binds to and inhibits BCL-X(L)-mediated protection against UV radiation-induced apoptosis.
Xue L, Chu F, Cheng Y, Sun X, Borthakur A, Ramarao M, Pandey P, Wu M, Schlossman SF, Prasad KV. Xue L, et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):6925-30. doi: 10.1073/pnas.102182299. Proc Natl Acad Sci U S A. 2002. PMID: 12011449 Free PMC article. - Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons.
Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA. Naumann T, et al. J Neurosci. 2002 Apr 1;22(7):2409-18. doi: 10.1523/JNEUROSCI.22-07-02409.2002. J Neurosci. 2002. PMID: 11923404 Free PMC article. - Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy.
Syroid DE, Maycox PJ, Soilu-Hänninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Syroid DE, et al. J Neurosci. 2000 Aug 1;20(15):5741-7. doi: 10.1523/JNEUROSCI.20-15-05741.2000. J Neurosci. 2000. PMID: 10908614 Free PMC article.
References
- Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. - PubMed
- Bamji SX, Miller FD. Comparison of the expression of a Ta1:nlacZ transgene and Ta1 α-tubulin mRNA in the mature central nervous system. J Comp Neurol. 1996;374:52–69. - PubMed
- Banner DW, D’Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kDa TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. - PubMed
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to trkA on PC12 cells. Neuron. 1994;13:203–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials