Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates - PubMed (original) (raw)
Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates
S Lambert et al. J Neurosci. 1997.
Abstract
AnkyrinG 480/270 kDa and three ankyrin-binding integral membrane proteins (neurofascin, NrCAM, and the voltage-dependent sodium channel) colocalize within a specialized domain of the spectrin-actin network found at axonal segments of nodes of Ranvier in myelinated axons. Before myelination in embryonic nerves, ankyrinG 480/270 kDa and the related ankyrin isoform ankyrinB 440 kDa are co-expressed along with NrCAM in an abundant, continuous distribution along the length of axons. This study has resolved intermediate stages in the developmental transition from a continuous distribution of ankyrinG 480/270 kDa in all axons to a highly polarized localization at the node of Ranvier in the developing rat sciatic nerve. The first detected event is formation of clusters containing the cell adhesion molecules neurofascin and NrCAM at sites independent of myelin-associated glycoprotein (MAG)-staining Schwann cell processes. Subsequent steps involve recruitment of ankyrinG 480/270 kDa and the voltage-dependent sodium channel to cluster sites containing cell adhesion molecules, and elaboration of MAG-staining Schwann cell processes adjacent to these cluster sites. Formation of the mature node of Ranvier results from the fusion of asynchronously formed pairs of clusters associated with MAG-positive Schwann cells flanking the site of presumed node formation. Studies with the hypomyelinating mutant mouse trembler demonstrate that the elaboration of compact myelin is not required for the formation of these clustered nodal intermediates. Clustering of neurofascin and NrCAM precedes redistribution of ankyrinG 480/270 kDa and the voltage-dependent sodium channel, suggesting that the adhesion molecules define the initial site for subsequent assembly of ankyrin and the voltage-dependent sodium channel.
Figures
Fig. 1.
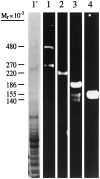
Immunoblot analysis of adult total rat brain membranes with antibodies against (1) ankyrinG 480/270 kDa, (2) voltage-dependent sodium channel, (3) neurofascin, and (4) NrCAM. _Lane 1′_shows the brain membrane preparation stained with Coomassie blue.
Fig. 2.
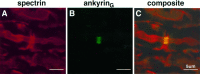
Immunofluorescence localization of ankyrinG 480/270 kDa with respect to spectrin at the node of Ranvier. A 4 μm cryosection of an adult rat sciatic nerve was double-labeled with an antibody against spectrin (A) and an antibody against ankyrinG480/270 kDa (B). The composite image (C) was collected by confocal microscopy.
Fig. 3.
Colocalization of ankyrinG 480/270 kDa, spectrin, NrCAM, and ankyrinB 440 kDa in dorsal root axons of the embryonic day 16 rat. Cryosections (4 μm) of the dorsal roots from an embryonic day 16 rat were double-labeled with antibodies to ankyrinG 480/270 kDa (B, D, F) and spectrin (A), NrCAM (C), or ankyrinB 440 kDa (E).Arrows indicate the staining of axons or bundles of axons emanating from the dorsal roots. Insets show a single bundle of axons at 2× higher magnification.
Fig. 4.
Localization of ankyrinG 480/270 kDa and ankyrinB 440 kDa with respect to MAG in the 2-d-old rat sciatic nerve. Cryosections (4 μm) of the 2-d-old rat sciatic nerve were double-labeled with antibodies to ankyrinG 480/270 kDa (A), ankyrinB 440 kDa (B), and MAG (C, D). Composite images collected by confocal microscopy are shown in _E_and F. Arrows denote the position of ankyrinG 480/270 kDa clusters. Inset shows one of these clusters at 3× higher magnification.
Fig. 5.
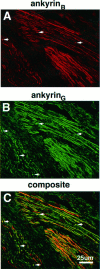
Colocalization of ankyrinG 480/270 kDa and ankyrinB 440 kDa in the 2-d-old rat sciatic nerve. A 4 μm cryosection of the 2-d-old rat sciatic nerve was labeled with antibodies to ankyrinB 440 kDa (A) and ankyrinG 480/270 kDa (B). The composite confocal image is shown in C.Arrows denote the position of ankyrinG480/270 kDa clusters.
Fig. 6.
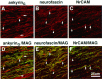
Co-clustering of ankyrinG and ankyrin-binding proteins in paired cluster intermediates of the 2-d-old rat sciatic nerve. Cryosections (4 μm) of the 2-d-old rat sciatic nerve were double-labeled with antibodies against ankyrinG(D–F) and the voltage-dependent sodium channel (A), neurofascin (B), or NrCAM (C). Composite confocal images of individual double-cluster structures are shown in_G–I_.
Fig. 7.
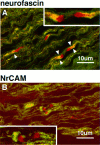
Immunolocalization of the ankyrin-binding cell adhesion molecules neurofascin and NrCAM with respect to MAG in the 5-d-old rat sciatic nerve. Confocal micrographs show 4 μm cryosections double-labeled with MAG (fluorescein) and neurofascin (rhodamine in A) or NrCAM (rhodamine in_B_). Arrowheads indicate areas of neurofascin and MAG overlap. Insets show areas of interest at 2× higher magnification.
Fig. 8.
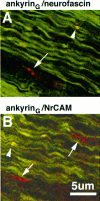
Colocalization of ankyrinG 480/270 kDa (fluorescein in A and B) with respect to neurofascin (rhodamine in A) and NrCAM (rhodamine in_B_) in 4 μm cryosections of the 2-d-old rat sciatic nerve. Co-clusters of ankyrinG 480/270 kDa with neurofascin or NrCAM are denoted by arrowheads. Clusters of neurofascin or NrCAM alone are denoted by_arrows_.
Fig. 9.
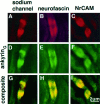
Distribution of ankyrinG 480/270 kDa (A, D), neurofascin (B, E), and NrCAM (C, F) in 4 μm cryosections of the 2-d-old sciatic nerve. Also shown is the localization of these proteins with respect to MAG in the same sections (D–F).Arrows denote clusters of proteins associated with MAG-positive processes; arrowheads show clusters of proteins not associated with MAG-positive processes.
Fig. 10.
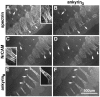
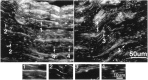
Immunolocalization of ankyrinG480/270 kDa to nodal intermediates in the 7-d-old rat sciatic nerve. Cryosections (4 μm) of the 7-d-old rat sciatic nerve were labeled with antibodies to ankyrinG 480/270 kDa.Arrows indicate nodal intermediates and are numbered to indicate progressive stages in node formation. Individual nodes from each potential intermediate stage are shown below at higher magnification.
Fig. 11.
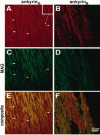
Immunolocalization of ankyrinG480/270 kDa, neurofascin, and the voltage-dependent sodium channel in sciatic nerves from the hypomyelinated mutant mouse_trembler_. Cryosections (4 μm) of sciatic nerves from 20-d-old wild-type (A–C) and_trembler_ (D–F) mice were stained with antibodies against ankyrinG 480/270 kDa (A, D), neurofascin (B, E), and the voltage-dependent sodium channel (C, F). Nodes of Ranvier are highlighted by arrows. Large arrowheads indicate double-cluster structures in the_trembler_ mutant similar to the nodal intermediates observed in the rat developing sciatic nerve. A′ and _D′_represent the corresponding DIC images of the immunofluorescent micrographs shown in A and D and illustrate the lack of compact myelin in the _trembler_mutant. Arrows (A′) indicate the positions of the nodes stained with the ankyrinG antibody in the wild-type sciatic nerve.
Similar articles
- Mechanisms of node of Ranvier assembly.
Rasband MN, Peles E. Rasband MN, et al. Nat Rev Neurosci. 2021 Jan;22(1):7-20. doi: 10.1038/s41583-020-00406-8. Epub 2020 Nov 25. Nat Rev Neurosci. 2021. PMID: 33239761 Review. - Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier.
Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR Jr, Peles E. Eshed Y, et al. Neuron. 2005 Jul 21;47(2):215-29. doi: 10.1016/j.neuron.2005.06.026. Neuron. 2005. PMID: 16039564 - The role of the ankyrin-binding protein NrCAM in node of Ranvier formation.
Custer AW, Kazarinova-Noyes K, Sakurai T, Xu X, Simon W, Grumet M, Shrager P. Custer AW, et al. J Neurosci. 2003 Nov 5;23(31):10032-9. doi: 10.1523/JNEUROSCI.23-31-10032.2003. J Neurosci. 2003. PMID: 14602817 Free PMC article. - Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels.
Bennett V, Lambert S. Bennett V, et al. J Neurocytol. 1999 Apr-May;28(4-5):303-18. doi: 10.1023/a:1007005528505. J Neurocytol. 1999. PMID: 10739573 Review.
Cited by
- Postnatal Loss of Neuronal and Glial Neurofascins Differentially Affects Node of Ranvier Maintenance and Myelinated Axon Function.
Taylor AM, Saifetiarova J, Bhat MA. Taylor AM, et al. Front Cell Neurosci. 2017 Feb 3;11:11. doi: 10.3389/fncel.2017.00011. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28217083 Free PMC article. - Mechanisms of node of Ranvier assembly.
Rasband MN, Peles E. Rasband MN, et al. Nat Rev Neurosci. 2021 Jan;22(1):7-20. doi: 10.1038/s41583-020-00406-8. Epub 2020 Nov 25. Nat Rev Neurosci. 2021. PMID: 33239761 Review. - Molecular mechanisms of node of Ranvier formation.
Susuki K, Rasband MN. Susuki K, et al. Curr Opin Cell Biol. 2008 Dec;20(6):616-23. doi: 10.1016/j.ceb.2008.09.007. Epub 2008 Nov 1. Curr Opin Cell Biol. 2008. PMID: 18929652 Free PMC article. Review. - Functional specialization of the axon initial segment by isoform-specific sodium channel targeting.
Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Boiko T, et al. J Neurosci. 2003 Mar 15;23(6):2306-13. doi: 10.1523/JNEUROSCI.23-06-02306.2003. J Neurosci. 2003. PMID: 12657689 Free PMC article. - Axon-dendrite and apical-basolateral sorting in a single neuron.
Lillis M, Zaccardi NJ, Heiman MG. Lillis M, et al. Genetics. 2022 May 5;221(1):iyac036. doi: 10.1093/genetics/iyac036. Genetics. 2022. PMID: 35244146 Free PMC article.
References
- Bennett V, Gilligan DM. The spectrin based membrane skeleton and micron scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
- Davis JQ, Bennett V. Brain spectrin. Isolation of subunits and formation of hybrids with erythrocyte spectrin subunits. J Biol Chem. 1983;258:7757–7766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials