Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse - PubMed (original) (raw)
Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse
M C Irizarry et al. J Neurosci. 1997.
Abstract
The PDAPP transgenic mouse overexpresses human amyloid precursor protein V717F (PDAPP minigene) and develops age-related cerebral amyloid-beta protein (Abeta) deposits similar to senile plaques in Alzheimer's disease. We find age-related cortical and limbic Abeta deposition that begins at 8 months and progresses to cover 20-50% of the neuropil in cingulate cortex, entorhinal cortex, and hippocampus of 18-month-old heterozygotic animals. The regional patterns of transgene expression and amyloid deposition suggest that Abeta deposits occur at the terminals of overexpressing neurons. Amyloid deposition is associated with dystrophic neurites and extensive gliosis. However, stereological analysis shows that there is no overt neuronal loss in entorhinal cortex, CA1 hippocampal subfield, or cingulate cortex through 18 months of age. In addition, there is no apparent loss of mRNA encoding neuronal synaptic, cytoskeletal, or metabolic proteins. Thus, widespread Abeta deposition in 18-month-old heterozygotic mice produces neuritic alterations and gliosis without widespread neuronal death.
Figures
Fig. 1.
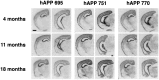
Expression of hAPP isoforms in PDAPP mice.In situ hybridization showing similar hAPP695, 751, and 770 mRNA regional distribution in anterior and posterior coronal sections at 4, 11, and 18 months of age in heterozygote transgenic mice. Anterior sections demonstrate strongest message in the hippocampus, followed by the cingulate and cortical regions, and minimal signal in hippocampal white matter and amygdala; posterior sections demonstrate in addition strong message in the superficial, but not deep, layers of the entorhinal cortex. Scale bar, 1 mm.
Fig. 2.
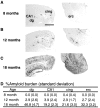
Amyloid burden in PDAPP mice. Immunostaining for Aβ demonstrates age-dependent accumulation of amyloid beginning in the cingulate cortex (cing) at 8 months (A), prominent in dentate gyrus (dg), CA1, and entorhinal cortex (erc) by 12 months (B), and profound deposition in these regions by 18 months (C), quantitated by percent amyloid burden (±SD) measurements in these regions (D). Scale bar, 1 mm.
Fig. 3.
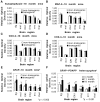
Neuron counts in PDAPP mice (±SD). No difference is noted in layer-by-layer neuronal counts in the entorhinal cortex of 8 (A), 12 (B), and 18 (C) month heterozygote transgenic mice compared with nontransgenic littermates. At 18 months, no significant difference is noted in the caudal CA1 or the caudal retrosplenial/cingulate region (D). Neuronal architecture is grossly preserved in wild-type mice (E) compared with nontransgenic animals (F) despite tremendous amyloid burden (G; Aβ immunostaining of section immediately adjacent to F). Counts reflect neurons in one hemisphere.cing, Cingulate cortex; erc, entorhinal cortex. Scale bar, 175 μm.
Fig. 4.
mRNA expression in PDAPP mice (±SD). There is no loss of mRNA for neuronal synaptic (synaptophysin, A), cytoskeletal (MAP-2, B), or metabolic (COX-2,C; COX-4, D) proteins in 18 month PDAPP heterozygote transgenic mice compared with nontransgenic littermates.In situ hybridization demonstrates significantly increased GFAP mRNA in heterozygote transgenic mice compared with nontransgenic littermates at 18 months of age (E) (p < 0.002 by ANOVA; individual region_t_ tests not reaching significance), increasing in an age-related fashion in the heterozygote transgenic mice (F). dg, Dentate gyrus granule cell layer; mol, molecular layer of dentate gyrus;cing, cingulate cortex; erc, entorhinal cortex; thal, thalamus. +, Post hoc p < 0.05, 18 versus 4 and 11 months; ++, post hoc p < 0.0,5 18 versus 4 months.
Fig. 5.
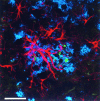
Confocal image demonstrating the association of Aβ deposition (blue) with GFAP-immunoreactive astrocytes (red) and hAPP immunoreactive structures (green) consistent with dystrophic neurites in an 18-month-old heterozygote PDAPP transgenic mouse. Scale bar, 25 μm.
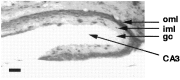
Fig. 6.
Immunostaining for Aβ in the hippocampus of an 18-month-old heterozygote transgenic mouse demonstrates the predisposition for Aβ deposition in the outer molecular layer (oml) of the dentate gyrus, which is the terminal zone for the projections of the perforant pathway. Scale bar, 100 μm.iml, Inner molecular layer; gc, granule cell layer.
Similar articles
- Cholinergic neuropathology in a mouse model of Alzheimer's disease.
German DC, Yazdani U, Speciale SG, Pasbakhsh P, Games D, Liang CL. German DC, et al. J Comp Neurol. 2003 Aug 4;462(4):371-81. doi: 10.1002/cne.10737. J Comp Neurol. 2003. PMID: 12811807 - Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease.
Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. Masliah E, et al. J Neurosci. 1996 Sep 15;16(18):5795-811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. J Neurosci. 1996. PMID: 8795633 Free PMC article. - Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging.
Nakagawa Y, Nakamura M, McIntosh TK, Rodriguez A, Berlin JA, Smith DH, Saatman KE, Raghupathi R, Clemens J, Saido TC, Schmidt ML, Lee VM, Trojanowski JQ. Nakagawa Y, et al. J Comp Neurol. 1999 Aug 30;411(3):390-8. J Comp Neurol. 1999. PMID: 10413774 - Alzheimer's disease and amyloid: culprit or coincidence?
Skaper SD. Skaper SD. Int Rev Neurobiol. 2012;102:277-316. doi: 10.1016/B978-0-12-386986-9.00011-9. Int Rev Neurobiol. 2012. PMID: 22748834 Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-β1-42 oligomers are revealed in vivo by using a novel animal model.
Brouillette J, Caillierez R, Zommer N, Alves-Pires C, Benilova I, Blum D, De Strooper B, Buée L. Brouillette J, et al. J Neurosci. 2012 Jun 6;32(23):7852-61. doi: 10.1523/JNEUROSCI.5901-11.2012. J Neurosci. 2012. PMID: 22674261 Free PMC article. - Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and alpha-synucleinopathies.
Goedert M. Goedert M. Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1101-18. doi: 10.1098/rstb.1999.0466. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434313 Free PMC article. Review. - Cholinergic dysfunction in a mouse model of Alzheimer disease is reversed by an anti-A beta antibody.
Bales KR, Tzavara ET, Wu S, Wade MR, Bymaster FP, Paul SM, Nomikos GG. Bales KR, et al. J Clin Invest. 2006 Mar;116(3):825-32. doi: 10.1172/JCI27120. Epub 2006 Feb 23. J Clin Invest. 2006. PMID: 16498501 Free PMC article. - Learning and memory deficits in APP transgenic mouse models of amyloid deposition.
Morgan D. Morgan D. Neurochem Res. 2003 Jul;28(7):1029-34. doi: 10.1023/a:1023255106106. Neurochem Res. 2003. PMID: 12737527 Review. - Amyloid-beta in Alzheimer's disease: the horse or the cart? Pathogenic or protective?
Lee HG, Castellani RJ, Zhu X, Perry G, Smith MA. Lee HG, et al. Int J Exp Pathol. 2005 Jun;86(3):133-8. doi: 10.1111/j.0959-9673.2005.00429.x. Int J Exp Pathol. 2005. PMID: 15910547 Free PMC article. Review.
References
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. - PubMed
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer disease. Neurology. 1992;42:631–639. - PubMed
- Braak H, Braak E. Neuropathological staging of Alzheimer related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
- Callahan LM, Coleman PD. Neurons bearing neurofibrillary tangles are responsible for selected synaptic deficits in Alzheimer’s disease. Neurobiol Aging. 1995;16:311–314. - PubMed
- Cavalieri B. Geometria degli indivisibili. Unione Tipografico; Torino, Italy: 1966.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous