Structural and functional analyses of human cerebral cortex using a surface-based atlas - PubMed (original) (raw)
Comparative Study
Structural and functional analyses of human cerebral cortex using a surface-based atlas
D C Van Essen et al. J Neurosci. 1997.
Abstract
We have analyzed the geometry, geography, and functional organization of human cerebral cortex using surface reconstructions and cortical flat maps of the left and right hemispheres generated from a digital atlas (the Visible Man). The total surface area of the reconstructed Visible Man neocortex is 1570 cm2 (both hemispheres), approximately 70% of which is buried in sulci. By linking the Visible Man cerebrum to the Talairach stereotaxic coordinate space, the locations of activation foci reported in neuroimaging studies can be readily visualized in relation to the cortical surface. The associated spatial uncertainty was empirically shown to have a radius in three dimensions of approximately 10 mm. Application of this approach to studies of visual cortex reveals the overall patterns of activation associated with different aspects of visual function and the relationship of these patterns to topographically organized visual areas. Our analysis supports a distinction between an anterior region in ventral occipito-temporal cortex that is selectively involved in form processing and a more posterior region (in or near areas VP and V4v) involved in both form and color processing. Foci associated with motion processing are mainly concentrated in a region along the occipito-temporal junction, the ventral portion of which overlaps with foci also implicated in form processing. Comparisons between flat maps of human and macaque monkey cerebral cortex indicate significant differences as well as many similarities in the relative sizes and positions of cortical regions known or suspected to be homologous in the two species.
Figures
Fig. 1.
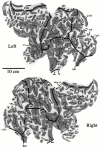
Image of the cut brain surface from the Visible Man cerebrum, taken 46 mm from the top of the head (26 mm from the beginning of cortex). A contour representing the estimated trajectory of cortical layer 4 is drawn for the left hemisphere. In regions where the cortex was cut obliquely, close scrutiny of several nearby sections was often needed to infer the most likely contour for layer 4. Cerebral cortex was contained in 108 images (1 mm intervals), with an in-plane resolution of 2048 × 1216 pixels (0.33 mm/pixel). The raw data coordinate system associated with this stack of images is represented by an x_–_y_-axis with origin at the_bottom left of each image. The section number relative to the topmost image indicates the value for the_z_-axis.
Fig. 8.
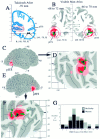
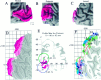
Stereotaxic projection of neuroimaging data to the cortical surface with estimation of spatial uncertainties.A, Activation foci from a study of motion analysis (Watson et al., 1993, their Table 3) are plotted on the nearest coronal section from the Talairach atlas (y = −70 mm). Black dots show the group means for the left and right hemisphere activation foci, which are located in white matter under the inferior temporal sulcus. B, The same foci are plotted in relation to coronal slices (5 mm thick) through the Visible Man atlas. Black dots show the group mean for each hemisphere; green dots show activation foci from individual subjects that intersect this slab of cortex. The _red_and pink circles are drawn at radii of 10 and 15 mm from the group means, respectively, and portions of the surface within each ring are shaded accordingly. C, E, Lateral views of the left and right hemispheres, respectively, showing where the foci and associated uncertainty zones are located in 3-D in and near the posterior inferior temporal sulcus (pITS). The pITS has also been identified as the ascending limb of the ITS byWatson et al. (1993). D, F, Flat maps of occipito-temporal cortex from the left and right hemispheres, respectively, showing where the group means (black dots) and individual values (green dots) project to the nearest point on the cortical surface and where the 10 and 15 mm uncertainty zones map in the vicinity of each group mean. Occlusion by overlying dots prevents some of the individual points from being visible. The activation foci for the group means have similar surface-based coordinates on the two cortical maps ([−158, +3]R-SB vs [−162, +2]L-SB). The maximum linear extent of each domain on the map is about twice the diameter of the corresponding 3-D sphere (∼45 map-mm for the 10 mm radius spheres, ∼60 map-mm for the 15 mm radius spheres).G, Histogram of the 3-D (RMS) distance between each individual focus and the group mean for that hemisphere from the data of Watson et al. (1993) (black bars) and for a corresponding data set from the fMRI study of motion processing by McCarthy et al. (1995), based on seven subjects (14 hemispheres).
Fig. 2.
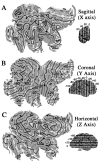
Surface reconstructions of the Visible Man. Lateral, medial, anterior, and posterior views are shown for both hemispheres. The origin was placed at the anterior commissure, the midsagittal plane was aligned to the y = 0 plane, and the anterior and posterior commissures were aligned to the_x_-axis. Native Visible Man (VM) and Talairach (T’88) coordinate systems are shown for each axis with tick marks at 1 cm intervals.Insets at the far left show the orientation of the original quasi-horizontal slices relative to the cardinal axes, with solid lines indicating 1 cm intervals. The maximum extent of the Visible Man surface is 68, 166, and 110 mm, respectively in the x, y, and_z_ dimensions for the left hemisphere and 68, 171, and 106 mm, respectively, for the right hemisphere. After transforming the Visible Man brain to Talairach space, these values are 3% larger in the x dimension, 1% larger in the z_dimension, and identical in the y dimension. After this transformation, the posterior pole of the Visible Man has a_y value of −107, compared with −106 of the Talairach brain, and the anterior pole has a y value of +59, identical to the +59 of the Talairach brain. Panels in the_middle_ show extensively smoothed surfaces for both hemispheres (500 iterations with a smoothing parameter of 0.5). These are shaded to reflect mean curvature of the original 3-D surface, with inward folds (fundi of sulci) shown in dark and outward folds (crests of gyri) in lighter shades. See Results and for abbreviations. We compared the locations in stereotaxic space of nine major sulci with those illustrated for a population of 20 normal brains by Steinmetz et al. (1990). In the left hemisphere, the trajectories are within the normal range for the central, precentral, postcentral, superior temporal, and calcarine sulci and for the Sylvian fissure and its posterior and anterior ascending rami. In the right hemisphere, the trajectories for these sulci are all within the normal range, except that the central, precentral, and postcentral sulci and the posterior ascending ramus of the Sylvian fissure were more posterior (by 3–10 mm) than in any of the cases illustrated by Steinmetz et al. (1990). Interestingly, the same sulci show a similar posterior displacement in the hemisphere illustrated in the atlas of Talairach and Tournoux (1988). Finally, the callosal sulcus in both the left and right hemispheres of the Visible Man appears to have a slightly abnormal shape, with the rostral extrema (genu of corpus callosum) slightly more posterior than normal and the superior margin slightly higher than normal.
Fig. 3.
Flat maps of the left and right cerebral hemispheres. Top panels show flat maps with mean curvature displayed to represent cortical geography. Each map was aligned by making the mean orientation of the fundus of the central sulcus on the flat map match the visually estimated average orientation of the lips of the central sulcus in the 3-D reconstruction. For a region that is folded but not intrinsically curved, a mean curvature of ±0.5 mm−1 (maximum on the scale) is equivalent to a cylinder of 1 mm radius. Middle panels show medial and lateral views of the intact hemispheres, with lobes identified according to landmarks delineated by Ono et al. (1990) and suitably colored (occipital lobe in pink, parietal lobe in_green_, temporal lobe in blue, frontal lobe in beige, and limbic lobe in_lavender_). C.C., Corpus callosum;HC, hippocampus; Amyg., amygdala; and_Olf._, olfactory cortex. _Bottom panels_show the same flat maps with lobes colored and with darker shading applied to all regions of buried cortex, i.e., cortex not externally visible in the intact hemisphere, as determined from the original image slices (compare Fig. 1) and from the 3-D surface and volume reconstructions. Black lines indicate sharply creased regions (fundi) within each sulcus that were traced manually on the curvature maps. The scale applies to all panels. Artificial cuts (blue lines) were introduced to reduce distortion in the flat maps.
Fig. 13.
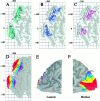
Interespecies comparisons between macaque and human cerebral cortex. A, 3-D surface reconstructions and a flat map of the macaque monkey (case 79-0; Drury et al., 1996a). The surface is colored to delineate the different cortical lobes, and shaded regions on the flat map indicate cortex buried within various sulci (abbreviations are a subset of those listed for Figure 5 (see ), except that_AS_ stands for arcuate sulcus; PS, the principal sulcus; and HF, the hippocampal fissure). The extent of different lobes in the macaque is based on designations byBonin and Bailey (1947) and Felleman and Van Essen (1991). Instead of making a cut along the V1/V2 boundary, as has been done for most previous cortical flat maps of the macaque (e.g., Van Essen and Maunsell, 1980; Drury et al., 1996a), a cut was made along the horizontal meridian representation in V1 (cf. Van Essen, 1997) to correspond better to the human flat map. Scale bars in A(and C) apply to the flat maps but not the 3-D views.B, 3-D reconstruction and cortical flat map of the Visible Man, modified from Figure 3. The more darkly shaded sulci in A and B are likely to correspond to one another, because they contain cortical areas that are known or likely to be homologous (see Results).C, Cortical areas in the macaque, according to the partitioning scheme of Felleman and Van Essen (1991). Note that the macaque map includes 3 cm2 of hippocampus and other archicortical and paleocortical structures, all limbic regions that were not included in the Visible Man reconstruction. As a basis for comparing surface geometry, we used the same indices as in Figure 4 and determined that the macaque cortex has about one-fourth of the intrinsic curvature of human cortex (ICI = 14 vs 55 for Visible Man) and one-third of the folding (FI = 160 vs 510 for Visible Man). D, Visual areas and functionally specialized visual regions displayed on the right hemisphere map of the Visible Man (adapted from Fig. 12_D_).
Fig. 4.
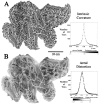
A, Intrinsic curvature of the cortical surface, displayed on a map of the right hemisphere. There are numerous regions of positive (spherical) curvature (dark) and of negative (saddle-shaped) curvature (light). A histogram of intrinsic curvature values is shown to the right. The mean value (0.004 mm−2) is slightly positive, reflecting the overall convex shape of the hemisphere. Only a small fraction of the cortical sheet (2% of total surface area) has an intrinsic curvature exceeding that of a sphere 4 mm in radius (i.e., intrinsic curvature >0.0625 mm−2). B, Areal distortion of the right hemisphere flat map. Dark and light regions represent tiles that are compressed or expanded, respectively, relative to their area in the 3-D reconstruction. A histogram of distortion ratios is shown to the right. The mean distortion ratio is 1.09, corresponding to an average of 9% greater surface area on the flat map compared with the corresponding area on the 3-D surface. For the left hemisphere map, the mean distortion ratio is 1.12; 6% of the tiles are expanded by more than 50% on the cortical map, and 2% of the tiles are compressed to an equivalent degree.
Fig. 5.
A geographical atlas showing sulci and gyri in the Visible Man. Sulcal and gyral abbreviations are listed in the, alphabetically for each lobe. Designations are based mainly on the atlases of Ono et al. (1990) and Jouandet et al. (1989). In cases of ambiguity or multiple terminology (usually in regions of high variability), we based our choice on the sulcal pattern that best matched the geography of the Visible Man. The pattern of convolutions in the Visible Man lies within the range of variability illustrated and analyzed by Ono et al. (1990) for a population of 25 brains.
Fig. 6.
Stereotaxic (Talairach) isocontours displayed on the Visible Man surface. Contours at 10 mm intervals in 3-D are displayed on flat maps for constant x(A), constant y(B), and constant z(C) values. For any point on the map, its Talairach coordinates can be determined by interpolation between contours on each panel. In the reverse direction, given a set of Talairach coordinates, the nearest point on the cortical map can be estimated by looking for intersection points on the appropriate isocontours.
Fig. 7.
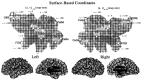
A surface-based coordinate system for the left hemisphere [_u, v_]L-SB and right hemisphere [_u, v_]R-SB of the Visible Man, displayed on a map of cortical geography. The origin corresponds to the ventral tip of the central sulcus, and grid lines are spaced at 20 map-mm on each map. The horizontal (u) axis extends from −253 to +170 map-mm for the left hemisphere and from −254 to +171 map-mm for the right hemisphere. The vertical (v) axis extends from −145 to +188 map-mm for the left hemisphere and from −134 to +174 for the right hemisphere. The bottom panels show lateral and medial views of the hemisphere, with the surface-based coordinate system wrapped up into 3-D space. The mean separation between adjacent resampled nodes in 3-D was 0.95 mm for the left hemisphere and 0.96 mm for the right hemisphere. This signifies an average linear expansion of 5% on the flat maps. Any point in the volume that lies above or below the surface can be represented in 3-D surface-based coordinates, using its distance from the surface in 3-D as one coordinate (w) and the nearest point on the surface for the other two coordinates ([_u, v, w_]R-SB or [_u, v, w_]L-SB, depending on the hemisphere). To determine the correspondence of the surface-based coordinates of major geographical landmarks, the centers of gravity (white dots) were determined for nine sulci: the central, postcentral, superior temporal, collateral, olfactory, fronto-orbital, and superior rostral sulci, plus dorsal and ventral halves of the calcarine sulcus (see Results and Table 2).
Fig. 9.
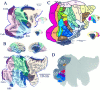
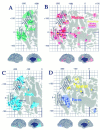
Estimated location and variability in extent of visual area V1 in the Visible Man. A–C, Medial, posterior, and lateral views of V1 determined from the postmortem architectonic study of Rademacher et al. (1993). Maroon_regions represent cortex that belongs to V1 in all or nearly all of the 20 hemispheres illustrated by Rademacher et al. (1993) in a series of medial and lateral drawings of each hemisphere.Red regions incorporate an additional belt of cortex that is part of V1 in about half of the hemispheres.Pink includes cortex that is part of V1 in a minority of cases, based on estimated distances from the margins of the calcarine sulcus and other geographical landmarks. D, The same inner, most likely, and outer border estimates for V1 are shown on a cortical flat map of the occipital lobe in relation to gyral and sulcal outlines. E, Two foci along the V1/V2 boundary taken from the fMRI mapping study of DeYoe et al. (1996) are shown in relation to a coronal slice through the Visible Man. The blue dot represents 8° eccentricity along the superior vertical meridian, and the blue ring indicates the 10 mm uncertainty zone surrounding this stereotaxic location. Similarly, the_green dot and ring represent 14° along the inferior vertical meridian. F, Projection of activation foci and associated 10 mm uncertainty zones for five eccentricities along the superior vertical meridian (blue dots and shading) and five eccentricities along the inferior vertical meridian (green dots and_shading_). Blue-green represents portions of the surface within 10 mm of both types of focus.
Fig. 10.
Boundaries of topographically organized visual areas on the Visible Man cortex estimated using surface-based and stereotaxic projection methods. A, Boundaries between V2/V3 (top of map) and V2/VP (bottom of map) shown in green, relative to the most likely V1/V2 boundary transferred from Figure 9 and shown in black.Green contours represent boundaries estimated on the basis of distances along the cortical surface, taken from the summary cortical maps of DeYoe et al. (1996) and Sereno et al. (1995).Green dots and shading represent specific eccentricities along these borders, the Talairach coordinates of which were determined using the isocontour maps of DeYoe et al. (1996) and then projected to the cortical surface along with associated 10 mm uncertainty zones. B, Boundaries between V3/V3A (top) and VP/V4v (bottom), estimated using the same strategies as in A. C, The horizontal meridian representation of V3A (top) and of V4v (bottom), again using the same methods. The estimated extent of areas V1 (red), V2 (yellow), V3 and VP (blue-green), and lower-field V3A and upper-field V4 (purple) are displayed on a flat map (D), a lateral view (E), and a medial view (F).
Fig. 11.
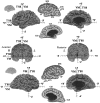
Distribution of activation sites associated with specific aspects of visual function. Each panel shows individual activation foci, identified according to the labels in Table 2, and cortex within the surrounding 10 mm uncertainty zone, for activations associated with processing of color (A, green), motion (B, red), form (inanimate objects or textures; C, light blue), faces (D, dark blue), and spatial relationships (D, yellow).
Fig. 12.
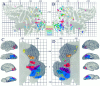
Combined analysis of activation foci for all foci listed in Table 3. Top panels, All 118 foci projected to flat maps of the left and right hemispheres. Note that activation foci originally reported for both the left hemisphere (x< 0 in Table 3, column 5) and the right hemisphere (_x_ > 0) are plotted on each map. When patterns were examined separately for data obtained from the left and right hemispheres, no pronounced hemispheric asymmetries were evident. Although not labeled on either map, individual foci can be identified by determining their surface-based coordinates and finding the corresponding focus in Table 3. The distribution of foci is generally similar on the two maps, but with a number of notable exceptions (see text). Note that on the left hemisphere map a few foci lie outside the perimeter of the map (blue dots near the parahippocampal gyrus on the bottom right). This is because of their distance and orientation relative to the nearest tile on the surface.Bottom panels, Estimated extent of cortex likely to be specialized for particular visual functions, including motion (M) in red, form (F) in blue, spatial analysis (S) in yellow, and of cortex involving overlapping or closely interdigitation of multiple functions, including form and color (FC) combined (blue-green); form and motion (FM) combined (purple); and motion, color, and spatial, (MCS) combined (orange). Results are displayed in three formats for each hemisphere, including lateral and ventral 3-D views, lateral and ventral smoothed views, and flat maps of the posterior half of each hemisphere. The total extent of visually responsive cortex estimated from the pattern of activation foci is shown in dark gray.
Similar articles
- Functional specializations in human cerebral cortex analyzed using the Visible Man surface-based atlas.
Drury HA, Van Essen DC. Drury HA, et al. Hum Brain Mapp. 1997;5:233-7. Hum Brain Mapp. 1997. PMID: 11542497 - Functional and structural mapping of human cerebral cortex: solutions are in the surfaces.
Van Essen DC, Drury HA, Joshi S, Miller MI. Van Essen DC, et al. Proc Natl Acad Sci U S A. 1998 Feb 3;95(3):788-95. doi: 10.1073/pnas.95.3.788. Proc Natl Acad Sci U S A. 1998. PMID: 9448242 Free PMC article. Review. - Functional analysis of V3A and related areas in human visual cortex.
Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Tootell RB, et al. J Neurosci. 1997 Sep 15;17(18):7060-78. doi: 10.1523/JNEUROSCI.17-18-07060.1997. J Neurosci. 1997. PMID: 9278542 Free PMC article. - Accurate prediction of V1 location from cortical folds in a surface coordinate system.
Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana Rosas H, Potthast A, Schwartz EL, Fischl B. Hinds OP, et al. Neuroimage. 2008 Feb 15;39(4):1585-99. doi: 10.1016/j.neuroimage.2007.10.033. Epub 2007 Nov 6. Neuroimage. 2008. PMID: 18055222 Free PMC article. - The representation of the ipsilateral visual field in human cerebral cortex.
Tootell RB, Mendola JD, Hadjikhani NK, Liu AK, Dale AM. Tootell RB, et al. Proc Natl Acad Sci U S A. 1998 Feb 3;95(3):818-24. doi: 10.1073/pnas.95.3.818. Proc Natl Acad Sci U S A. 1998. PMID: 9448246 Free PMC article. Review.
Cited by
- Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature.
Destrieux C, Fischl B, Dale A, Halgren E. Destrieux C, et al. Neuroimage. 2010 Oct 15;53(1):1-15. doi: 10.1016/j.neuroimage.2010.06.010. Epub 2010 Jun 12. Neuroimage. 2010. PMID: 20547229 Free PMC article. - Diffeomorphic metric surface mapping in subregion of the superior temporal gyrus.
Vaillant M, Qiu A, Glaunès J, Miller MI. Vaillant M, et al. Neuroimage. 2007 Feb 1;34(3):1149-59. doi: 10.1016/j.neuroimage.2006.08.053. Epub 2006 Dec 19. Neuroimage. 2007. PMID: 17185000 Free PMC article. - Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases.
Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Van Essen DC, et al. Cereb Cortex. 2012 Oct;22(10):2241-62. doi: 10.1093/cercor/bhr291. Epub 2011 Nov 2. Cereb Cortex. 2012. PMID: 22047963 Free PMC article. - Intrinsic curvature: a marker of millimeter-scale tangential cortico-cortical connectivity?
Ronan L, Pienaar R, Williams G, Bullmore E, Crow TJ, Roberts N, Jones PB, Suckling J, Fletcher PC. Ronan L, et al. Int J Neural Syst. 2011 Oct;21(5):351-66. doi: 10.1142/S0129065711002948. Int J Neural Syst. 2011. PMID: 21956929 Free PMC article. - A bipolar taxonomy of adult human brain sulcal morphology related to timing of fetal sulcation and trans-sulcal gene expression gradients.
Snyder WE, Vértes PE, Kyriakopoulou V, Wagstyl K, Williams LZJ, Moraczewski D, Thomas AG, Karolis VR, Seidlitz J, Rivière D, Robinson EC, Mangin JF, Raznahan A, Bullmore ET. Snyder WE, et al. bioRxiv [Preprint]. 2023 Dec 20:2023.12.19.572454. doi: 10.1101/2023.12.19.572454. bioRxiv. 2023. PMID: 38168226 Free PMC article. Updated. Preprint.
References
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey. I. Cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. - PubMed
- Anderson CH, Drury HA, Lee CW, Carman GJ, Van Essen DC. Computerized reconstruction and flattening of macaque cerebral cortex. Soc Neurosci Abstr. 1994;20:428.
- Andreasen NC, Arndt S, Swayze V, II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt C, Yuh WTC. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. - PubMed
- Bonin GV, Bailey P. The neocortex of Macaca mulatta. University of Illinois; Urbana, IL: 1947.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources