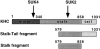Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis - PubMed (original) (raw)
Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis
G Q Bi et al. J Cell Biol. 1997.
Abstract
Kinesin and myosin have been proposed to transport intracellular organelles and vesicles to the cell periphery in several cell systems. However, there has been little direct observation of the role of these motor proteins in the delivery of vesicles during regulated exocytosis in intact cells. Using a confocal microscope, we triggered local bursts of Ca2+-regulated exocytosis by wounding the cell membrane and visualized the resulting individual exocytotic events in real time. Different temporal phases of the exocytosis burst were distinguished by their sensitivities to reagents targeting different motor proteins. The function blocking antikinesin antibody SUK4 as well as the stalk-tail fragment of kinesin heavy chain specifically inhibited a slow phase, while butanedione monoxime, a myosin ATPase inhibitor, inhibited both the slow and fast phases. The blockage of Ca2+/calmodulin-dependent protein kinase II with autoinhibitory peptide also inhibited the slow and fast phases, consistent with disruption of a myosin-actin- dependent step of vesicle recruitment. Membrane resealing after wounding was also inhibited by these reagents. Our direct observations provide evidence that in intact living cells, kinesin and myosin motors may mediate two sequential transport steps that recruit vesicles to the release sites of Ca2+-regulated exocytosis, although the identity of the responsible myosin isoform is not yet known. They also indicate the existence of three semistable vesicular pools along this regulated membrane trafficking pathway. In addition, our results provide in vivo evidence for the cargo-binding function of the kinesin heavy chain tail domain.
Figures
Figure 1
Primary sequence of KHC. Arrows indicate approximate sites for antibody recognition. Numbers refer to KHC amino acid sequence number starting from the amino terminus. The “Stalk-Tail” and “Stalk” are the two KHC fragments used in this experiment.
Figure 2
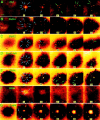
Effect of reagents targeted against motor proteins on Ca2+-regulated exocytosis induced by laser wounding. The pseudocolor pictures are confocal fluorescence images of extracellular rhodamine dextran showing the exocytotic pockets into confocal focal plane just under the plasma membrane (Bi et al., 1995). Exocytotic events are visualized as the appearance of bright disks (0.5–1 μm diameter) against the dark intracellular background indicated by arrows. Blue arrows indicate early events, while green arrows indicate later events. (A) The exocytotic pattern of a sea urchin embryonic cell in natural sea water. In other series, the embryos were previously injected with (B) SUK4, (C) SUK2 antikinesin, (D) stalk-tail fragment, and (E) stalk fragment of KHC and were kept in natural sea water. In series F, the embryo was treated with 50 mM BDM. In series G, the embryo was injected with CaMK(273-302), an autoinhibitory peptide from the regulatory domain of CaM kinase type II. The large central stain in G is dye entering the unhealed wound. The first frame in each series was collected before wounding. Time 0 is defined as the moment immediately after wounding. The unit for time labels is seconds. Arrows indicate new exocytotic events that occurred since the previous frame. Bar, 5 μm.
Figure 3
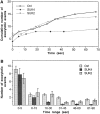
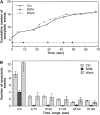
Specific inhibition of a slow phase of Ca2+-regulated exocytosis by function-blocking antikinesin antibody SUK4. A quantifies cumulative number of exocytotic events in individual cells from typical experiments. The control cell (Ctrl) was not injected with any reagent. B summarizes the average number of exocytotic events that occurred within different time ranges from n experiments. n = 37 for Ctrl, 17 for SUK4, and 23 for SUK2. A biphasic temporal pattern of exocytosis is seen by comparing the SUK4 injection data with the Ctrl or SUK2 injection data. The slow phase (after 16 s), but not the fast phase (0–15 s) of exocytosis is inhibited by SUK4 antikinesin. Error bars are standard errors.
Figure 4
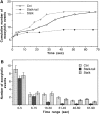
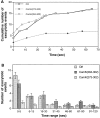
Specific inhibition of the slow phase of Ca2+-regulated exocytosis by KHC stalk-tail fragment. (A) Typical examples of the cumulative number of exocytotic events in individual cells. (B) The average number of exocytotic events that occurred within different time ranges from n experiments. n = 37 for Ctrl, 27 for Stalk-tail, and 33 for Stalk.
Figure 5
Reversible inhibition of both the slow and the fast phases of exocytosis by BDM. The cells were in 50 mM BDM for 10–60 min. For “Wash” experiments, cells were in 50 mM BDM for at least 45 min and were then transferred to BDM-free sea water for at least 15 min before imaging. (A) Quantified examples of individual experiments under different conditions. (B) Average number of exocytotic events that occurred within different time ranges from n experiments. n = 37 for Ctrl, 17 for BDM, and 8 for Wash.
Figure 6
Inhibition of both the slow and the fast phases of exocytosis by CaMK(273-302), an autoinhibitory peptide from the regulatory domain of CaM kinase type II. Control peptide CaMK(284-302) did not inhibit exocytosis. (A) Typical examples of cumulative number of exocytotic events in individual cells. (B) The average number of exocytotic events that occurred within different time ranges from n experiments. n = 18 for Ctrl, 39 for CaMK(273-302), and 22 for CaMK(284-302).
Figure 7
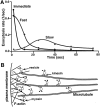
Three distinct vesicle pools and two-step vesicular recruitment for Ca2+-regulated exocytosis. (A) Three vesicle pools and their temporal distribution of exocytosis rates (number of exocytotic events per unit time) based on the data shown in Figs. 3–5. The “Immediate” pool of vesicles (squares) are BDM insensitive (and also kinesin reagents insensitive) and are therefore not dependent of either kinesin or myosin transport mechanism. The “Fast” pool (diamonds) is myosin dependent but kinesin independent. It was calculated by subtracting the myosin-independent (BDM-insensitive) component from the kinesin-independent exocytosis (average of SUK4 and Stalk-tail data). The “Slow” pool (circles) is kinesin dependent and was obtained by subtracting the kinesin-independent exocytosis from the average of control, SUK2, and Stalk data. (B) The proposed relative distribution of different vesicle pools and the kinesin- and myosin-mediated transport mechanisms.
Similar articles
- Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release.
Steinhardt RA, Bi G, Alderton JM. Steinhardt RA, et al. Science. 1994 Jan 21;263(5145):390-3. doi: 10.1126/science.7904084. Science. 1994. PMID: 7904084 - Vesicle transport: the role of actin filaments and myosin motors.
DePina AS, Langford GM. DePina AS, et al. Microsc Res Tech. 1999 Oct 15;47(2):93-106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10523788 Review. - The role of myosin in vesicle transport during bovine chromaffin cell secretion.
Neco P, Gil A, Del Mar Francés M, Viniegra S, Gutiérrez LM. Neco P, et al. Biochem J. 2002 Dec 1;368(Pt 2):405-13. doi: 10.1042/BJ20021090. Biochem J. 2002. PMID: 12225290 Free PMC article. - Myosin motors and not actin comets are mediators of the actin-based Golgi-to-endoplasmic reticulum protein transport.
Durán JM, Valderrama F, Castel S, Magdalena J, Tomás M, Hosoya H, Renau-Piqueras J, Malhotra V, Egea G. Durán JM, et al. Mol Biol Cell. 2003 Feb;14(2):445-59. doi: 10.1091/mbc.e02-04-0214. Mol Biol Cell. 2003. PMID: 12589046 Free PMC article. - Molecular motors involved in chromaffin cell secretion.
Rosé SD, Lejen T, Casaletti L, Larson RE, Pene TD, Trifaró JM. Rosé SD, et al. Ann N Y Acad Sci. 2002 Oct;971:222-31. doi: 10.1111/j.1749-6632.2002.tb04466.x. Ann N Y Acad Sci. 2002. PMID: 12438122 Review.
Cited by
- Clonal tests of conventional kinesin function during cell proliferation and differentiation.
Brendza RP, Sheehan KB, Turner FR, Saxton WM. Brendza RP, et al. Mol Biol Cell. 2000 Apr;11(4):1329-43. doi: 10.1091/mbc.11.4.1329. Mol Biol Cell. 2000. PMID: 10749933 Free PMC article. - Short-term potentiation of membrane resealing in neighboring cells is mediated by purinergic signaling.
Togo T. Togo T. Purinergic Signal. 2014;10(2):283-90. doi: 10.1007/s11302-013-9387-y. Purinergic Signal. 2014. PMID: 24122144 Free PMC article. - Cell membrane disruption stimulates NO/PKG signaling and potentiates cell membrane repair in neighboring cells.
Togo T. Togo T. PLoS One. 2012;7(8):e42885. doi: 10.1371/journal.pone.0042885. Epub 2012 Aug 7. PLoS One. 2012. PMID: 22880128 Free PMC article. - Analysis of cytoskeletal and motility proteins in the sea urchin genome assembly.
Morris RL, Hoffman MP, Obar RA, McCafferty SS, Gibbons IR, Leone AD, Cool J, Allgood EL, Musante AM, Judkins KM, Rossetti BJ, Rawson AP, Burgess DR. Morris RL, et al. Dev Biol. 2006 Dec 1;300(1):219-37. doi: 10.1016/j.ydbio.2006.08.052. Epub 2006 Aug 26. Dev Biol. 2006. PMID: 17027957 Free PMC article. - Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin.
Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Twelvetrees AE, et al. Neuron. 2010 Jan 14;65(1):53-65. doi: 10.1016/j.neuron.2009.12.007. Neuron. 2010. PMID: 20152113 Free PMC article.
References
- Adams RJ, Pollard TD. Propulsion of organelles isolated from Acanthamoeba along actin filaments by myosin-I. Nature (Lond) 1986;322:754–756. - PubMed
- Allan V. Membrane traffic motors. FEBS Lett. 1995;369:101–106. - PubMed
- Bennett MK, Scheller RH. Molecular correlates of synaptic vesicle docking and fusion. Curr Opin Neurobiol. 1994;4:324–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous