Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages - PubMed (original) (raw)
Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages
D J Hackam et al. J Exp Med. 1997.
Abstract
Phagocytosis of bacteria by macrophages and neutrophils is an essential component of host defense against infection. The mechanism whereby the interaction of opsonized particles with Fcgamma receptors triggers the engulfment of opsonized particles remains incompletely understood, although activation of tyrosine kinases has been recognized as an early step. Recent studies in other systems have demonstrated that tyrosine kinases can in turn signal the activation of small GTPases of the ras superfamily. We therefore investigated the possible role of Rho in Fc receptor-mediated phagocytosis. To this end we microinjected J774 macrophages with C3 exotoxin from Clostridium botulinum, which ADP-ribosylates and inactivates Rho. C3 exotoxin induced the retraction of filopodia, the disappearance of focal complexes, and a global decrease in the F-actin content of J774 cells. In addition, these cells exhibited increased spreading and the formation of vacuolar structures. Importantly, inactivation of Rho resulted in the complete abrogation of phagocytosis. Inhibition of Fcgamma receptor-mediated phagocytosis by C3 exotoxin was confirmed in COS cells, which become phagocytic upon transfection of the FcgammaRIIA receptor. Rho was found to be essential for the accumulation of phosphotyrosine and of F-actin around phagocytic cups and for Fcgamma receptor-mediated Ca2+ signaling. The clustering of receptors in response to opsonin, an essential step in Fcgamma-induced signaling, was the earliest event shown to be inhibited by C3 exotoxin. The effect of the toxin was specific, since clustering and internalization of transferrin receptors were unaffected by microinjection of C3. These data identify a role for small GTPases in Fcgamma receptor-mediated phagocytosis by leukocytes.
Figures
Figure 1
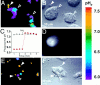
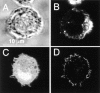
Determination of phagocytosis in J774 and FcγRIIA-transfected COS-1 cells. J774 macrophages (A–D) or FcγRIIA-transfected COS cells (E and F) were incubated with fluoresceinated, IgG-opsonized zymosan particles, some of which became internalized. (A) The fluorescence emitted by the particles was measured with alternating excitation at 440 nm and 490 nm, and the fluorescence ratio was used to estimate the pH at the particle surface. A pseudocolor image is shown in A, corresponding to the pH scale shown to the right. Extracellular particles (solid arrowheads) display higher pH values than internalized particles (open arrowheads). (B) The cells in A as visualized using differential interference contrast (Nomarski) optics. (C) Time course of pH changes observed in an alkaline (likely extracellular; open white circles) and an acidic (likely intraphagosomal; solid red circles) particle. Where indicated, 50 mM NH4Cl was added to the medium, producing alkalinization of internalized particles. (D) The left hand cell in A and B underwent control microinjection with the injection marker, fura-dextran, dissolved in injection buffer alone. The fluorescence emission of fura (excitation: 360 nm; emission: 535 nm) is illustrated. (E) Pseudocolor image of the fluorescence ratio of IgG-opsonized zymosan particles incubated with FcγRIIA-transfected COS-1 cells. Extracellular particles (solid arrowheads) display higher pH values than internalized particles (open arrowheads). (F) The cells in A as visualized using differential interference contrast optics. Representative of at least five separate experiments of each type.
Figure 2
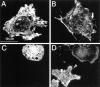
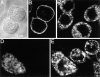
The effect of microinjected C3 exotoxin on the actin cytoskeleton of FcγRIIA-transfected COS and J774 cells. (A) FcγRIIA-transfected COS cells were mock-injected with the fluorescent dye Lucifer yellow dissolved in injection buffer alone, fixed, and stained with rhodamine phalloidin to visualize F-actin. Note the presence of stress fibers and actin accumulation at adhesion plaques. (B) FcγRIIA-transfected COS cells were injected with C3 exotoxin 60 min before fixation and staining with rhodamine phalloidin. Note the disappearance of stress fibers and focal adhesions and the persistence of F-actin along membrane ruffles. (C) Lucifer yellow emission of J774 cells, identifying the top cell as having been injected with C3 exotoxin. (D) Rhodamine-phalloidin staining of the cells shown in C. Note the decrease in F-actin content, and the absence of filopodia (open arrowhead) and focal complexes (solid arrowhead) in the C3-injected cell (top right) compared to the uninjected (bottom left) cells. Representative of eight separate experiments.
Figure 3
The effect of Rho inhibition on cell morphology and phagocytosis. (A–C) J774 cells were plated on glass and some were injected with C3 exotoxin in a solution containing fura-dextran as a marker. The cells were then incubated with fluoresceinated, opsonized zymosan. (A) Differential interference contrast micrograph. Note the morphological alterations of the left hand, injected cell. (B) Fluorescence emission of the cells shown in A (excitation 360 nm; emission 510 nm). The emission of fura-dextran, demonstrating that only the left hand cell was injected. The emission of fluorescein, which can be seen despite the low excitation wavelength, reveals the labeled zymosan particles. (C) Effect of C3 exotoxin on phagocytosis by cells plated on glass, determined as described in Materials and Methods. Data are means ± SE of 175 control and 160 C3- injected cells (P <0.05.). (D–F) J774 cells were plated on fibronectin and some were injected with C3 exotoxin as above. The cells were then incubated with fluoresceinated, opsonized zymosan. (D) Differential interference contrast micrograph. (E) Fluorescence emission of the cells shown in D. (F) Effect of C3 exotoxin on phagocytosis on fibronectin. Data are means ± SE of 150 control and 74 C3-injected cells (P <0.05). (G–I) FcγRIIA receptor–transfected COS cells were plated on glass coverslips and some were injected with C3 exotoxin as above. The cells were then incubated with fluoresceinated, opsonized zymosan. (G) Differential interference contrast micrograph. (H) Fluorescence emission of the cells shown in G. (I) Effect of C3 exotoxin on phagocytosis by transfected COS cells. Data are means ± SE of 165 control and 75 C3-injected cells (P <0.05). Images are represented of five separate experiments.
Figure 4
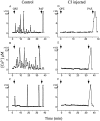
Effect of Rho on Fcγ receptor–mediated increases in [Ca2+]i. J774 cells, some of which had been microinjected with C3 exotoxin 1 h before, were loaded with fura-2. The concentration of [Ca2+]i was measured by ratio imaging as described in Materials and Methods. Where indicated, the cells were exposed to opsonized zymosan (OPZ) or to PAF (1 μM). Injected cells were identified by the fluorescence of the injection marker, fluorescein dextran. The changes in [Ca2+]i from eight separate cells are shown. Control cells : A–C. C3-injected cells: D–F. Uninjected cells exhibited transient, asynchronous rises in [Ca2+]i in response to opsonized zymosan and a synchronous rise in response to PAF. By contrast, C3-injected cells did not exhibit changes in [Ca2+]i in response to zymosan, yet responded to PAF. Representative of five separate experiments with at least eight cells/group.
Figure 5
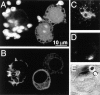
Effect of C3 microinjection on the accumulation of F-actin and phosphotyrosine at nascent phagocytic cups. Fluoresceinated, opsonized zymosan particles were added for 30 min to J774 cells, some of which had been injected with C3 exotoxin and Lucifer yellow 1 h before. (A–D) Confocal fluorescence images. (A) Green fluorescence emission showing the particles and identifying the injected cells. Note that the two right hand cells were injected, and bound fewer particles compared with the uninjected cell (left hand) (B) Red fluorescence illustrating the distribution of F-actin, stained with rhodamine-phalloidin. Note the F-actin cups in uninjected cells but not in C3-injected cells. (C) Immunostaining of a control cell with antiphosphotyrosine antibody. The accumulation of phosphotyrosine at phagocytic cups is illustrated. (D) Antiphosphotyrosine staining of a C3-injected cell, identified by the emission of Lucifer yellow (not shown). Note the absence of phosphotyrosine accumulation at sites where latex particles adhered (see E). (E) Differential interference contrast micrograph of the cell in D. Images are representative of five experiments of at least 20 cells/experiment.
Figure 6
Quantification of Fcγ receptors on the surface of control and C3-injected cells. J774 cells were injected with C3 exotoxin and the fluorescent dye (Lucifer yellow) 1 h before the determination of Fcγ receptor expression using anti-FcγII/III receptor antibodies and confocal microscopy. (A) Lucifer yellow fluorescence, demonstrating that the upper right hand cell had been injected. (B) Corresponding confocal micrograph illustrating the abundance and distribution of Fcγ receptors. (C) Quantification of relative fluorescence intensity of cells stained with anti-FcγII/III receptor antibodies, as in B. Data are means ± SE of 75 control and 53 C3-treated cells.
Figure 7
Effect of Rho on Fcγ receptor clustering. (A) Differential interference contrast image of a J774 cell which had been exposed to anti-Fcγ receptor antibody and to secondary antibody in the cold, followed by incubation for 5 min at 37°C to induce cross-linking of Fcγ receptors. (B) Confocal micrograph illustrating the Fcγ receptor distribution of the cell in A. Note the polar distribution of receptors. (C) Lucifer yellow emission identifying a cell which had been injected with C3 exotoxin 1 h before receptor cross-linking, which was performed as in A. (D) Confocal micrograph illustrating the Fcγ receptor distribution of the cell in C. Note that receptor clustering did not occur (compare B and D). Representative of five separate experiments of at least 20 cells/experiment.
Figure 8
Transferrin internalization in J774 cells: effect of C3 exotoxin. (A) Differential interference contrast image of J774 cells which had been exposed to Texas red–labeled transferrin for 1 h at 4°C. (B) The cells in A were analyzed by confocal fluorescence microscopy to detect the distribution of transferrin. (C) Cells were initially incubated with Texas red–labeled transferrin as in A and then warmed to 37°C for another hour to promote receptor internalization. A typical confocal micrograph is illustrated. (D and E) Selected cells were injected with C3 exotoxin and Lucifer yellow (as a marker of microinjection) and the whole cell population was then allowed to bind and internalize Texas red–labeled transferrin as in C. (D) Lucifer yellow emission identifying a cell which had been injected with C3 exotoxin ∼1 h before exposure to transferrin. (E) Confocal micrograph illustrating the distribution of transferrin in the same cells as shown in D. Note that internalization occurred similarly in both injected cells (left hand cell in E) and noninjected cells. Representative of three separate experiments, with at least 25 injected cells/experiment.
Similar articles
- PAG3/Papalpha/KIAA0400, a GTPase-activating protein for ADP-ribosylation factor (ARF), regulates ARF6 in Fcgamma receptor-mediated phagocytosis of macrophages.
Uchida H, Kondo A, Yoshimura Y, Mazaki Y, Sabe H. Uchida H, et al. J Exp Med. 2001 Apr 16;193(8):955-66. doi: 10.1084/jem.193.8.955. J Exp Med. 2001. PMID: 11304556 Free PMC article. - Inhibition of Fcgamma receptor-mediated phagocytosis by a nonphagocytic Fcgamma receptor.
Hunter S, Indik ZK, Kim MK, Cauley MD, Park JG, Schreiber AD. Hunter S, et al. Blood. 1998 Mar 1;91(5):1762-8. Blood. 1998. PMID: 9473244 - A requirement for ARF6 in Fcgamma receptor-mediated phagocytosis in macrophages.
Zhang Q, Cox D, Tseng CC, Donaldson JG, Greenberg S. Zhang Q, et al. J Biol Chem. 1998 Aug 7;273(32):19977-81. doi: 10.1074/jbc.273.32.19977. J Biol Chem. 1998. PMID: 9685333 - Bacterial inhibition of phagocytosis.
Ernst JD. Ernst JD. Cell Microbiol. 2000 Oct;2(5):379-86. doi: 10.1046/j.1462-5822.2000.00075.x. Cell Microbiol. 2000. PMID: 11207593 Review. - Tyrosine phosphorylation and Fcgamma receptor-mediated phagocytosis.
Strzelecka A, Kwiatkowska K, Sobota A. Strzelecka A, et al. FEBS Lett. 1997 Jan 2;400(1):11-4. doi: 10.1016/s0014-5793(96)01359-2. FEBS Lett. 1997. PMID: 9000504 Review.
Cited by
- Cdc42 and RhoB activation are required for mannose receptor-mediated phagocytosis by human alveolar macrophages.
Zhang J, Zhu J, Bu X, Cushion M, Kinane TB, Avraham H, Koziel H. Zhang J, et al. Mol Biol Cell. 2005 Feb;16(2):824-34. doi: 10.1091/mbc.e04-06-0463. Epub 2004 Dec 1. Mol Biol Cell. 2005. PMID: 15574879 Free PMC article. - Differential endocytotic characteristics of a novel human B/DC cell line HBM-Noda: effective macropinocytic and phagocytic function rather than scavenging function.
Torii I, Morikawa S, Nagasaki M, Nokano A, Morikawa K. Torii I, et al. Immunology. 2001 May;103(1):70-80. doi: 10.1046/j.1365-2567.2001.01218.x. Immunology. 2001. PMID: 11380694 Free PMC article. - Monosodium urate-crystal-stimulated phospholipase D in human neutrophils.
Marcil J, Harbour D, Houle MG, Naccache PH, Bourgoin S. Marcil J, et al. Biochem J. 1999 Jan 15;337 ( Pt 2)(Pt 2):185-92. Biochem J. 1999. PMID: 9882614 Free PMC article. - Regulation of Microglial Phagocytosis by RhoA/ROCK-Inhibiting Drugs.
Scheiblich H, Bicker G. Scheiblich H, et al. Cell Mol Neurobiol. 2017 Apr;37(3):461-473. doi: 10.1007/s10571-016-0379-7. Epub 2016 May 13. Cell Mol Neurobiol. 2017. PMID: 27178562 Free PMC article. - Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr.
Gresham HD, Dale BM, Potter JW, Chang PW, Vines CM, Lowell CA, Lagenaur CF, Willman CL. Gresham HD, et al. J Exp Med. 2000 Feb 7;191(3):515-28. doi: 10.1084/jem.191.3.515. J Exp Med. 2000. PMID: 10662797 Free PMC article.
References
- Greenberg S., and S.C. Silverstein. 1993. Phagocytosis. In Fundamental Immunology. W.E. Paul, editor. Raven Press, Inc., New York. 941–964.
- Swenson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–94. - PubMed
- Cox D, Chang P, Kurosaki T, Greenberg S. Syk tyrosine kinase is required for immunoreceptor tyrosine activation motif-dependent actin assembly. J Biol Chem. 1996;271:16597–16602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous