Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits - PubMed (original) (raw)
Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits
K S Chen et al. J Neurosci. 1997.
Abstract
Administration of nerve growth factor (NGF) to aged or lesioned animals has been shown to reverse the atrophy of basal forebrain cholinergic neurons and ameliorate behavioral deficits. To examine the importance of endogenous NGF in the survival of basal forebrain cholinergic cells and in spatial memory, mice bearing a disruption mutation in one allele of the NGF gene were studied. Heterozygous mutant mice (ngf+/-) have reduced levels of NGF mRNA and protein within the hippocampus and were found to display significant deficits in memory acquisition and retention in the Morris water maze. The behavioral deficits observed in NGF-deficient mice were accompanied by both shrinkage and loss of septal cells expressing cholinergic markers and by a decrease in cholinergic innervation of the hippocampus. Infusions of NGF into the lateral ventricle of adult ngf+/- mice abolished the deficits on the water maze task. Prolonged exposure to NGF may be required to induce cognitive effects, because reversal of the acquisition deficit was seen after long (5 weeks) but not short (3 d) infusion. Although NGF administration did not result in any improvement in the number of septal cells labeled for choline acetyltransferase, this treatment did effectively correct the deficits in both size of cholinergic neurons and density of cholinergic innervation of the hippocampus. These findings demonstrate the importance of endogenous NGF for survival and function of basal forebrain cholinergic neurons and reveal that partial depletion of this trophic factor is associated with measurable deficits in learning and memory.
Figures
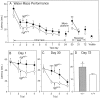
Fig. 1.
A, Mean latency to find the hidden platform on the water maze task on each day of testing. Each_point_ represents the mean performance of a group of mice on the four trials per day. Days 1–8 = performance during the acquisition of the initial location of the hidden platform; day 24 = performance on testing for retention of the initial platform location after a 16 d delay; days_30_ and 31 = performance during the acquisition of a new platform location; day_72_ = performance on testing for retention of the new platform location after a 6 week delay. The _point_labeled Visible represents performance on the visible platform task. B, Mean latency to find the hidden platform on each of the individual four trials on Day 1, and (C) on Day 30.D, Mean latency to find the hidden platform on the first trial of Day 72 (*p < 0.05, **p < 0.01, *** p < 0.0005; one-factor ANOVA with post hoc Fisher PLSD).
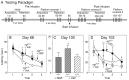
Fig. 2.
A, Testing paradigm of the second study, which assessed the effects of exogenous administration of NGF.B, Mean latency to find the hidden platform on the water maze task on the first day of acquisition after intraventricular infusions (Day 68). * denotes a significant difference between the two ngf+/− groups and ngf+/+ group; p < 0.05. C, Mean latency to find the hidden platform on the first trial of the first day of retention of this new platform location (Day 100).D, Mean latency to find the hidden platform during an acquisition task conducted on Day 103, 5 weeks after the onset of NGF infusion. * denotes a difference between NGF-infused and vehicle-infused ngf+/− mice (one-factor ANOVA with_post hoc_ Fisher PLSD).
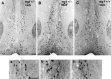
Fig. 3.
Photomicrograph of the medial septal region of (A) a ngf+/− mouse that had received vehicle, (B) a ngf+/− mouse that had received NGF, and (C) a_ngf_+/+ mouse that had received vehicle stained with antibodies to ChAT; insets show a higher magnification of stained cells. Scale bar, 100 μm.
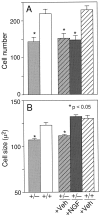
Fig. 4.
Number (A) and size (B) of ChAT-positive cells in the medial septal region [* denotes a significant difference from either untreated or vehicle-treated wild-type mice (p < 0.05; one-factor ANOVA with post hoc Fisher PLSD)].
Fig. 5.
Photomicrograph of the CA1 region of the hippocampus of (A) a ngf+/− mouse that had received vehicle, (B) a_ngf_+/− mouse that had received NGF, and (C) a ngf+/+ mouse that had received vehicle stained with AChE. Scale bar, 100 μm.
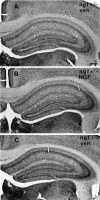
Fig. 6.
Photomicrograph of the hippocampus of (A) a ngf+/− mouse that had received vehicle, (B) a ngf+/− mouse that had received NGF, and (C) a_ngf_+/+ mouse that had received vehicle stained with AChE. Scale bar, 100 μm.
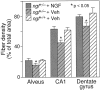
Fig. 7.
Density of AChE-positive fibers in the alveus, CA1, and dentate gyrus regions in the hippocampus [* denotes a significant difference between vehicle-treated ngf+/− and either ngf+/+ or NGF-treated ngf+/− mice (p < 0.05; one-factor ANOVA with_post hoc_ Fisher PLSD].
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
- Aloe L, Cozzari C, Calissano P, Levi-Montalcini R. Somatic and behavioral postnatal effects of fetal injections of nerve growth factor antibodies in the rat. Nature. 1981;291:413–415. - PubMed
- Chen KS, Masliah E, Mallory M, Terry RD, Gage FH. Synaptic loss in cognitively impaired aged rats is ameliorated by chronic human nerve growth factor infusion. Neuroscience. 1995;68:19–27. - PubMed
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases