Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel alpha subunit - PubMed (original) (raw)
Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel alpha subunit
A R Cantrell et al. J Neurosci. 1997.
Abstract
Phosphorylation of brain Na+ channel alpha subunits by cAMP-dependent protein kinase (PKA) decreases peak Na+ current in cultured brain neurons and in mammalian cells and Xenopus oocytes expressing cloned brain Na+ channels. We have studied PKA regulation of Na+ channel function by activation of D1-like dopamine receptors in acutely isolated hippocampal neurons using whole-cell voltage-clamp recording techniques. The D1 agonist SKF 81297 reversibly reduced peak Na+ current in a concentration-dependent manner. No changes in the voltage dependence or kinetics of activation or inactivation were observed. This effect was mediated by PKA, as it was mimicked by application of the PKA activator Sp-5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole-3', 5'-monophosphorothioate(cBIMPS) and was inhibited by the specific PKA inhibitor peptide PKAI5-24. cBIMPS had similar effects on type IIA brain Na+ channel alpha subunits expressed in tsA-201 cells, but no effect was observed on a mutant Na+ channel alpha subunit in which serine residues in five PKA phosphorylation sites in the intracellular loop connecting domains I and II (LI-II) had been replaced by alanine. A single mutation, S573A, similarly eliminated cBIMPS modulation. Thus, activation of D1-like dopamine receptors results in PKA-dependent phosphorylation of specific sites in LI-II of the Na+ channel alpha subunit, causing a reduction in Na+ current. Such modulation is expected to exert a profound influence on overall neuronal excitability. Dopaminergic input to the hippocampus from the mesocorticolimbic system may exert this influence in vivo.
Figures
Fig. 1.
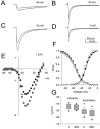
D1-like dopamine receptor agonists reduce whole-cell Na+ current. A, The absolute value of peak inward Na+ current is plotted as a function of time during the experiment. The cell was exposed to SKF 81297 (5 μ
m
) by rapid perfusion during the period indicated by the solid bar. Test pulses to −20 mV (40 msec long) were applied once every 2 sec from a holding potential of −75 mV. The same pulse protocol was used in subsequent figures unless otherwise indicated. B, Representative current traces obtained under control conditions (1) and in the presence of SKF 81297 (2) recorded at the times indicated in A. Inset, Box plot showing the extent of current reduction in cells that responded to SKF 81297.C, Effect of SKF 38393 (10 μ
m
) on peak inward Na+ current as described in A.D, Representative current traces obtained under control conditions (1) and in the presence of SKF 38393 (2) at the times indicated in C.Inset, Box plot showing the extent of reduction in cells that responded to SKF 38393.
Fig. 2.
Involvement of a D1-like dopamine receptor in the signaling pathway. A, Dose–response curve for SKF 81297 reduction in current. The solid line is a least squares fit of a Langmuir isotherm to the data with_K_I = 745 n
m
.Inset, Box plot summarizing similar data from eight independent experiments. B, Representative current traces from the cell in A obtained in control and in the presence of the indicated SKF 81297 concentrations. C, Peak Na+ current as a function of time as described for Figure 1_A_. The cell was first exposed to 10 μ
m
SCH 23390, then to a mixture of SCH 23390 and 10 μ
m
SKF 81297, and finally to SKF 81297 alone, as indicated by the bars. D, Representative current traces from the experiment shown in C.
Fig. 3.
Voltage dependence of D1-like dopamine receptor effect on Na+ currents. A–D, Whole-cell Na+ current elicited by test pulses to the indicated potentials from a holding potential of −75 mV in the absence and presence of 5 μ
m
SKF 81297. In each panel, the smaller current was obtained in the presence of SKF 81297.E, Current–voltage relationship for peak Na+ current in control (closed squares) and in the presence of SKF 81297 (closed circles). The SKF 81297 points have been scaled to facilitate comparison of the voltage dependence of the Na+current in the absence and presence of the compound (shaded triangle). F, Boltzmann fits of the activation and inactivation curves for the same cell in control (closed symbols) and in the presence of SKF 81297 (open symbols). G, Box plot showing the range of half-activation and half-inactivation voltages obtained in control and in the presence of SKF 81297.
Fig. 4.
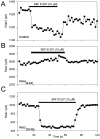
Reduction of Na+ current by PKA activation. A, Peak Na+ currents elicited in control and in the presence of 50 μ
m
cBIMPS plotted as a function of time during the experiment. B, Representative current traces in control (1) and in the presence of cBIMPS (2) recorded at the times indicated in A. Inset, Box plot showing the extent of current reduction in response to cBIMPS.C, Peak Na+ currents plotted as a function of time. The cell was first exposed to 5 μ
m
SKF 81297, then 50 μ
m
cBIMPS, and finally to a mixture of SKF 81297 and cBIMPS as indicated by the bars. The time course was corrected for a 20% rundown in the currents by subtraction of an exponential fit to the currents during control periods.D, Representative current traces in cBIMPS (2) and in the presence of cBIMPS plus SKF 81297 (3) recorded at the times indicated in_C_.
Fig. 5.
Block of D1-like dopaminergic Na+ current modulation by an inhibitor of PKA. Modulation of peak Na+ current by 10 μ
m
SKF 81297 with a control pipette solution (A) and using pipette solutions containing the PKA inhibitor PKAI5–24 (B) or the PKC inhibitor PKCI19–36 (C). Experimental procedures were as described in Figure1_A_.
Fig. 6.
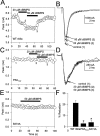
Modulation by PKA activation requires direct phosphorylation of the Na+ channel α subunit.A, tsA-201 cell expressing WT rat brain type IIA α subunits was exposed to cBIMPS (20–50 μ
m
) during the periods indicated by the solid bars. B, Representative current traces obtained under control conditions (1) and in the presence of cBIMPS (2, 3) at the times indicated in A. The absolute value of peak inward Na+ current is plotted as a function of time during the experiment. C, A tsA-201 cell expressing expressing mutant PKATOT was exposed to cBIMPS (20–50 μ
m
) during the periods indicated by the_solid bars_. The absolute value of peak inward Na+ current is plotted as a function of time during the experiment. D, Representative current traces obtained under control conditions (1) and in the presence of cBIMPS (2, 3) at the times indicated in_C_. E, A tsA-201 cell expressing expressing mutant S573A was exposed to cBIMPS (50 μ
m
) during the periods indicated by the solid bar. The absolute value of peak inward Na+ current is plotted as a function of time during the experiment. F, Bar graphs showing the response to 50 μ
m
cBIMPS in tsA-201 cells expressing WT, PKATOT, or S573A mutant Na+ channels.
Similar articles
- Dopaminergic modulation of voltage-gated Na+ current in rat hippocampal neurons requires anchoring of cAMP-dependent protein kinase.
Cantrell AR, Tibbs VC, Westenbroek RE, Scheuer T, Catterall WA. Cantrell AR, et al. J Neurosci. 1999 Sep 1;19(17):RC21. doi: 10.1523/JNEUROSCI.19-17-j0003.1999. J Neurosci. 1999. PMID: 10460275 Free PMC article. - Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons.
Cantrell AR, Scheuer T, Catterall WA. Cantrell AR, et al. J Neurosci. 1999 Jul 1;19(13):5301-10. doi: 10.1523/JNEUROSCI.19-13-05301.1999. J Neurosci. 1999. PMID: 10377341 Free PMC article. - Molecular mechanism of convergent regulation of brain Na(+) channels by protein kinase C and protein kinase A anchored to AKAP-15.
Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Cantrell AR, et al. Mol Cell Neurosci. 2002 Sep;21(1):63-80. doi: 10.1006/mcne.2002.1162. Mol Cell Neurosci. 2002. PMID: 12359152 - Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.
Catterall WA. Catterall WA. Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review. - Oncogenes and signal transduction pathways involved in the regulation of Na+ channel expression.
Sashihara S, Tsuji S, Matsui T. Sashihara S, et al. Crit Rev Oncog. 1998;9(1):19-34. doi: 10.1615/critrevoncog.v9.i1.20. Crit Rev Oncog. 1998. PMID: 9754445 Review.
Cited by
- Text mining neuroscience journal articles to populate neuroscience databases.
Crasto CJ, Marenco LN, Migliore M, Mao B, Nadkarni PM, Miller P, Shepherd GM. Crasto CJ, et al. Neuroinformatics. 2003;1(3):215-37. doi: 10.1385/NI:1:3:215. Neuroinformatics. 2003. PMID: 15046245 - Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits.
Gao WJ, Krimer LS, Goldman-Rakic PS. Gao WJ, et al. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):295-300. doi: 10.1073/pnas.98.1.295. Proc Natl Acad Sci U S A. 2001. PMID: 11134520 Free PMC article. - Structure and function of voltage-gated sodium channels.
Marban E, Yamagishi T, Tomaselli GF. Marban E, et al. J Physiol. 1998 May 1;508 ( Pt 3)(Pt 3):647-57. doi: 10.1111/j.1469-7793.1998.647bp.x. J Physiol. 1998. PMID: 9518722 Free PMC article. Review. - Regulation of sodium channel activity by phosphorylation.
Scheuer T. Scheuer T. Semin Cell Dev Biol. 2011 Apr;22(2):160-5. doi: 10.1016/j.semcdb.2010.10.002. Epub 2010 Oct 13. Semin Cell Dev Biol. 2011. PMID: 20950703 Free PMC article. Review. - Regulation of membrane excitability: a convergence on voltage-gated sodium conductance.
Lin WH, Baines RA. Lin WH, et al. Mol Neurobiol. 2015 Feb;51(1):57-67. doi: 10.1007/s12035-014-8674-0. Epub 2014 Mar 29. Mol Neurobiol. 2015. PMID: 24677068 Free PMC article. Review.
References
- Arnt J, Hyttel J, Sánchez C. Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioral effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol. 1992;213:259–267. - PubMed
- Bernardo LS, Prince DA. Dopamine modulates a Ca2+ activated potassium conductance in mammalian hippocampal pyramidal cells. Nature. 1982;297:76–79. - PubMed
- Berretta N, Berton F, Bianchi R, Capogna M, Francesconi W, Brunelli M. Effects of dopamine, D-1 and D-2 dopaminergic agonists on the excitability of hippocampal CA1 pyramidal cells in guinea pig. Exp Brain Res. 1990;83:124–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials