Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus - PubMed (original) (raw)
Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus
R Shigemoto et al. J Neurosci. 1997.
Abstract
Neurotransmission in the hippocampus is modulated variously through presynaptic metabotropic glutamate receptors (mGluRs). To establish the precise localization of presynaptic mGluRs in the rat hippocampus, we used subtype-specific antibodies for eight mGluRs (mGluR1-mGluR8) for immunohistochemistry combined with lesioning of the three major hippocampal pathways: the perforant path, mossy fiber, and Schaffer collateral. Immunoreactivity for group II (mGluR2) and group III (mGluR4a, mGluR7a, mGluR7b, and mGluR8) mGluRs was predominantly localized to presynaptic elements, whereas that for group I mGluRs (mGluR1 and mGluR5) was localized to postsynaptic elements. The medial perforant path was strongly immunoreactive for mGluR2 and mGluR7a throughout the hippocampus, and the lateral perforant path was prominently immunoreactive for mGluR8 in the dentate gyrus and CA3 area. The mossy fiber was labeled for mGluR2, mGluR7a, and mGluR7b, whereas the Schaffer collateral was labeled only for mGluR7a. Electron microscopy further revealed the spatial segregation of group II and group III mGluRs within presynaptic elements. Immunolabeling for the group III receptors was predominantly observed in presynaptic active zones of asymmetrical and symmetrical synapses, whereas that for the group II receptor (mGluR2) was found in preterminal rather than terminal portions of axons. Target cell-specific segregation of receptors, first reported for mGluR7a (Shigemoto et al,., 1996), was also apparent for the other group III mGluRs, suggesting that transmitter release is differentially regulated by 2-amino-4-phosphonobutyrate-sensitive mGluRs in individual synapses on single axons according to the identity of postsynaptic neurons.
Figures
Fig. 1.
Immunoblot analysis of rat hippocampus (A) and receptor-expressing cells (B). Crude membrane preparations from rat hippocampus and CHO cells expressing mGluR4a (4a), mGluR7a (7a), or mGluR7b (7b), or COS cells transfected with mGluR8 cDNA (8) were subjected to 7% SDS-PAGE and transferred onto PVDF filters.A, The filters with the hippocampus were reacted with antibody to pan mGluR1 (G18), mGluR1α (A52), mGluR2/3 (H12), mGluR2 (mG2Na-5), mGluR4a (K44), mGluR5 (G53), mGluR7a (G71), mGluR7b (K74), or mGluR8 (K88). Immunoreactive bands for mGluR7b were completely abolished by preadsorption of the antibody with the corresponding peptide (adsorbed). B, The filters with the receptor-expressing cells were reacted with the mGluR7a (G71), mGluR7b (K74), mGluR4a (K44), or mGluR8 (K88) antibody. Each of the immunoreactive products was absent in nontransfected cells (not shown). Positions of molecular mass markers (Bio-Rad) in kDa are indicated on the left.
Fig. 2.
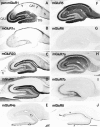
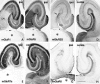
Distribution of immunoreactivity for eight mGluRs in rat hippocampus. Parasagittal sections through the hippocampus were reacted with antibody to pan mGluR1 (A), mGluR1α (B), mGluR2/3 (C), mGluR2 (D), mGluR4a (E), mGluR5 (F), mGluR6 (G), mGluR7a (H), mGluR7b (I), or mGluR8 (J). Immunoreactivity for mGluR5 (F) and mGluR7a (H) is distributed in all dendritic layers throughout the hippocampus, whereas immunoreactivity for the other mGluRs is restricted to distinct regions. Immunoreactivity for mGluR1 (A) is strong in dendritic fields of the dentate gyrus (DG) and CA3, as well as in the CA1 stratum oriens/alveus border (arrows). Immunoreactivity for mGluR1α (B) is strong only in the CA1 stratum oriens/alveus border (see Results). Immunoreactivity for mGluR2/3 (C) and mGluR2 (D) is strong in terminal zones of the perforant path and mossy fibers (see Results), whereas that for mGluR7b (I) is restricted to the mossy fiber terminal zone, and that for mGluR8 (J) to the lateral perforant path terminal zone, i.e., the outer third (filled arrowheads) of the dentate molecular layer and outer layer (double arrowhead) of CA3 stratum lacunosum moleculare. Immunoreactivity for mGluR4a (E) is weak but prominent in the inner third (open arrows) of the molecular layer. Labeling for mGluR6 (G) is hardly detected in the hippocampus. The rectangle in_C_ indicates a corresponding region shown with a higher magnification in Figure 3. fi, Fimbria;S, subiculum. Scale bar, 500 μm.
Fig. 3.
Distribution of immunoreactivity for mGluR1 (A), mGluR5 (B), mGluR2/3 (C), and mGluR7a (D) in the hippocampal area corresponding to the rectangle in Figure2_C_. Immunoreactivity for mGluR1 and mGluR5 is relatively uniform throughout the dentate molecular layer (Mo), whereas that for mGluR2/3 and mGluR7a is prominent in the middle one-third of the layer (mid), which is the terminal zone of the medial perforant path. In the hilus (Hi), dendritic profiles are immunopositive for mGluR1, mGluR5, and mGluR7a, whereas neuropil is immunopositive for mGluR2/3. Dendritic fields (stars) of CA3 pyramidal cells (Py) are immunopositive for mGluR1, mGluR5, and mGluR7a but immunonegative for mGluR2/3. Intense mGluR2/3 labeling is seen in CA1 stratum lacunosum moleculare (LM). The _broken line_indicates the hippocampal fissure. Gr, Granule cell layer; in, inner one-third of the molecular layer;out, outer one-third of the molecular layer. Scale bar, 100 μm.
Fig. 4.
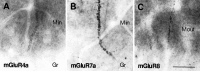
High-magnification micrographs showing immunoreactivity for mGluR4a (A), mGluR7a (B), and mGluR8 (C) in the inner (A, B) and outer (C) third of the dentate molecular layer. Note dendritic profiles decorated with puncta immunopositive for mGluRs in the inner (Min) and outer (Mout) third of the molecular layer.Gr, Granule cell layer. Scale bar, 25 μm.
Fig. 5.
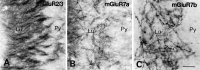
Immunoreactivity for mGluR2/3 (A), mGluR7a (B), and mGluR7b (C) in CA3 stratum lucidum. Axon bundle-like profiles are immunopositive for mGluR2/3, whereas punctate decorations of dendrites are immunopositive for mGluR7a and mGluR7b in stratum lucidum (Lu). The pyramidal cell layer (Py) is devoid of immunoreactivity. Scale bar, 25 μm.
Fig. 6.
Double-immunofluorescence study for mGluR7b, mGluR1α, and mGluR7a in the hilus and CA3 stratum lucidum. Fluorescence micrographs of sections double-immunolabeled for mGluR7b and mGluR1α (A, A’) or mGluR7b and mGluR7a (B, B’) were taken from identical fields of the hilus (A, A’) and CA3 (B, B’) under different filters. Punctate immunolabeling for mGluR7b (A, visualized with Texas Red) decorates mGluR1α-immunopositive interneurons (A’, visualized with fluorescein) in the hilus (Hi). In CA3, all profiles decorated with mGluR7b (B) are also decorated with mGluR7a immunoreactivity (B’, visualized with fluorescein) in stratum lucidum (Lu). Stratum radiatum (Ra) is immunopositive only for mGluR7a (B’). Arrows indicate cell bodies decorated with mGluR7a/b and labeled for mGluR1α immunoreactivity.Gr, Granule cell layer; Py, pyramidal cell layer. Scale bar, 30 μm.
Fig. 7.
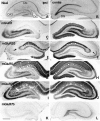
Distribution of immunoreactivity for mGluRs in the ipsilateral (A–C, E, G) and contralateral (D, F, H) hippocampus on the seventh day after placing a massive unilateral lesion in the perforant path. Adjacent horizontal sections were reacted with antibodies to mGluR1 (A), mGluR5 (B), mGluR2/3 (C, D), mGluR7a (E, F), and mGluR8 (G, H). The lesion (asterisks) involves the entorhinal cortex and subiculum. Marked reduction of immunoreactivity for mGluR2/3 (C), mGluR7a (E), and mGluR8 (G) is observed in the ipsilateral perforant path terminal zones of the CA areas, i.e., stratum lacunosum moleculare (arrowheads in_E_) and the molecular layer of the dentate gyrus (DG). No apparent changes are found in immunoreactivity for mGluR1 or mGluR5 (A, B). An mGluR2/3 immunoreactive area remains (arrow in C) in CA1 stratum lacunosum moleculare near CA1–CA2 transition. ipsi, Ipsilateral to the lesion; contra, contralateral to the lesion. Scale bar, 500 μm.
Fig. 8.
Effects of unilateral colchicine injection into the hilus on immunoreactivity for mGluRs in the ipsilateral (A, C, E, G, I, K) and contralateral (B, D, F, H, J, L) hippocampus. Adjacent frontal sections were reacted with antibody to mGluR1 (C, D), mGluR2/3 (E, F), mGluR5 (G, H), mGluR7a (I, J), or mGluR7b (K, L). Massive degeneration of granule cells in the dentate gyrus (DG) ipsilateral to the colchicine injection is seen in Nissl-stained sections (A, B). On the seventh day after the colchicine injection, marked reduction of immunoreactivity for mGluR1 (C) and mGluR5 (G) is observed in the dentate molecular layer ipsilateral to the lesion, whereas marked reduction of immunoreactivity for mGluR2/3 (arrowhead in E), mGluR7a (arrows in I), and mGluR7b (K) is observed in the ipsilateral mossy fiber terminal zone. Scale bar, 500 μm.
Fig. 9.
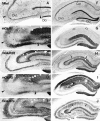
Effects of small unilateral injection (A–E) and bilateral intraventricular injection (F–J) of kainate on immunoreactivity for mGluRs in the hippocampus. A small amount of kainate (0.2 μg in 0.2 μl of PBS) was injected into two small areas in CA3, resulting in degeneration of CA3 pyramidal cells in the restricted areas as shown with Nissl staining (arrowheads in A). A larger amount of kainate (0.6 μg in 1.2 μl of PBS) was injected into bilateral ventricles to make massive bilateral degeneration of CA3 pyramidal cells, with most CA1 pyramidal cells being preserved (F). Adjacent frontal sections were reacted with antibodies to mGluR1 (B, G), mGluR2/3 (C, H), mGluR5 (D, I), or mGluR7a (E, J). On the seventh day after placing the lesions, immunoreactivity for mGluR1 and mGluR5 is reduced in dendritic fields corresponding to the lesions (arrowheads in B and D; CA3 area in G and I), whereas that for mGluR2/3 and mGluR7a shows no changes after the small injection (C, E). After the bilateral intraventricular kainate injection, reduction of mGluR2/3 immunoreactivity is apparent in CA1 stratum lacunosum moleculare (arrows in_H_), whereas that of mGluR7a immunoreactivity is marked not only in the same layer (arrows in_J_) but also in strata radiatum (Ra) and oriens (Or) of both CA1 and CA3. Interneurons immunoreactive to mGluR1 (see Results; compare Fig.2_A_) also disappear completely in the CA1 stratum oriens/alveus border (open arrows in G).DG, Dentate gyrus; LM, stratum lacunosum moleculare; Lu, stratum lucidum. Scale bars: 250 μm for A–E; 500 μm for F–J.
Fig. 10.
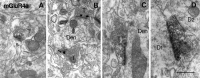
Electron micrographs showing immunoreactivity for mGluR2/3 (A, C, D, E) and mGluR2 (B) in CA1 stratum lacunosum moleculare (A–C), CA3 stratum lucidum (D), and CA1 stratum radiatum (E) as detected by preembedding immunoperoxidase (A, B, D, E) and immunogold (C) methods. A, Peroxidase reaction product for mGluR2/3 is accumulated intracellularly in a preterminal axon (arrows), which is continuous to an unlabeled axon terminal making asymmetrical synapses (arrowheads).B, Peroxidase reaction product for mGluR2 is accumulated extracellularly along axons and axon terminals (double arrowheads), but not in the synaptic clefts of asymmetrical synapses (arrowheads). C1, C2, and_C3_ were taken from three serial sections. Silver-enhanced immunogold particles for mGluR2/3 are found along a preterminal axon (arrows), which is continuous to an axon terminal making unlabeled asymmetrical synapses (arrowheads).Open and closed stars indicate corresponding regions in the serial sections. D, Peroxidase labeling for mGluR2/3 is observed in mossy fibers (arrows), one of which (asterisk) is continuous to a giant mossy fiber terminal (MT) making unlabeled asymmetrical synapses (arrowhead) with spines of CA3 pyramidal cells. E, Glial processes are also labeled for mGluR2/3. Scale bar, 0.5 μm.
Fig. 11.
Electron micrographs showing immunoreactivity for mGluR4a in the inner third of the dentate molecular layer (A, C, D) and in CA2 stratum oriens (B). Peroxidase (A, C, D) and immunogold (B) labeling for mGluR4a is found in presynaptic membrane specialization of asymmetrical synapses (A, B) on spines (s) and dendrites (Den) or symmetrical synapses (C, D) on dendrites (Den, D1). The labeled axon terminal (asterisk in_D_) in symmetrical synaptic contact with _D1_makes another unlabeled asymmetrical synapse on another dendrite (D2). T, Axon terminal. Scale bar, 0.5 μm.
Fig. 12.
Electron micrographs showing immunoreactivity for mGluR7a (A, B) and mGluR7b (C–E) in CA1 stratum radiatum (A), hilus (B), and CA3 stratum lucidum (C–E). Peroxidase reaction product for mGluR7a is accumulated along presynaptic membrane specialization of asymmetrical synapses on spines (s) of CA1 pyramidal cells (A) and symmetrical synapses on a soma (So) in the hilus (B). Immunogold particles for mGluR7b are concentrated in presynaptic membrane specialization of asymmetrical synapses on dendrites and necks of long spines (C). Symmetrical synapses on a dendrite (Den) are also labeled for mGluR7b (D). Note that the accumulation of the peroxidase reaction product is restricted to active zones of presynaptic membrane (D). E, A giant mossy fiber terminal (MT) makes a labeled synapse on a dendritic profile (asterisk) of a presumed interneuron and also makes unlabeled synapses on spines (s) of CA3 pyramidal cells. T, Axon terminal. Scale bars: 0.5 μm for A, B; 0.26 μm for C, D; 0.4 μm for E.
Fig. 13.
Electron micrographs showing immunoreactivity for mGluR8 in the dentate molecular layer (A, B, D) and CA2 stratum oriens (C). Peroxidase reaction product is accumulated in axon terminals, which make asymmetrical synapses on spines (s in A) or dendrites (Den in B; D1 in_D_) or a symmetrical synapse on a soma (_So_in C). Inset in B_indicates immunogold labeling for mGluR8 concentrated in presynaptic membrane specialization. Most of the asymmetrical synapses on the dendritic profile are labeled in B. The axon terminal in_D makes a labeled asymmetrical synapse on a dendrite (D1) and an unlabeled asymmetrical synapse on another dendrite (D2). Scale bar, 0.5 μm.
Similar articles
- High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus.
Somogyi P, Dalezios Y, Luján R, Roberts JD, Watanabe M, Shigemoto R. Somogyi P, et al. Eur J Neurosci. 2003 Jun;17(12):2503-20. doi: 10.1046/j.1460-9568.2003.02697.x. Eur J Neurosci. 2003. PMID: 12823458 - Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites.
Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Tamaru Y, et al. Neuroscience. 2001;106(3):481-503. doi: 10.1016/s0306-4522(01)00305-0. Neuroscience. 2001. PMID: 11591452 - The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations.
Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. Petralia RS, et al. Neuroscience. 1996 Apr;71(4):949-76. doi: 10.1016/0306-4522(95)00533-1. Neuroscience. 1996. PMID: 8684625 - mGluRs modulate strength and timing of excitatory transmission in hippocampal area CA3.
Cosgrove KE, Galván EJ, Barrionuevo G, Meriney SD. Cosgrove KE, et al. Mol Neurobiol. 2011 Aug;44(1):93-101. doi: 10.1007/s12035-011-8187-z. Epub 2011 May 11. Mol Neurobiol. 2011. PMID: 21559753 Review.
Cited by
- Activation of group II metabotropic glutamate receptors blocks zinc release from hippocampal mossy fibers.
Matias CM, Dionísio JC, Saggau P, Quinta-Ferreira ME. Matias CM, et al. Biol Res. 2014 Dec 18;47(1):73. doi: 10.1186/0717-6287-47-73. Biol Res. 2014. PMID: 25723955 Free PMC article. - Glutamate-dependent neuroglial calcium signaling differs between young and adult brain.
Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Sun W, et al. Science. 2013 Jan 11;339(6116):197-200. doi: 10.1126/science.1226740. Science. 2013. PMID: 23307741 Free PMC article. - Correlative analysis of immunoreactivity in confocal laser-scanning microscopy and scanning electron microscopy with focused ion beam milling.
Sonomura T, Furuta T, Nakatani I, Yamamoto Y, Unzai T, Matsuda W, Iwai H, Yamanaka A, Uemura M, Kaneko T. Sonomura T, et al. Front Neural Circuits. 2013 Feb 25;7:26. doi: 10.3389/fncir.2013.00026. eCollection 2013. Front Neural Circuits. 2013. PMID: 23443927 Free PMC article. - Functional monoclonal antibody acts as a biased agonist by inducing internalization of metabotropic glutamate receptor 7.
Ullmer C, Zoffmann S, Bohrmann B, Matile H, Lindemann L, Flor P, Malherbe P. Ullmer C, et al. Br J Pharmacol. 2012 Dec;167(7):1448-66. doi: 10.1111/j.1476-5381.2012.02090.x. Br J Pharmacol. 2012. PMID: 22747985 Free PMC article. - Synthesis, radiolabelling and in vitro and in vivo evaluation of a novel fluorinated ABP688 derivative for the PET imaging of metabotropic glutamate receptor subtype 5.
Sephton SM, Dennler P, Leutwiler DS, Mu L, Wanger-Baumann CA, Schibli R, Krämer SD, Ametamey SM. Sephton SM, et al. Am J Nucl Med Mol Imaging. 2012;2(1):14-28. Epub 2011 Dec 10. Am J Nucl Med Mol Imaging. 2012. PMID: 23133799 Free PMC article.
References
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. - PubMed
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system, second edition. Academic; San Diego: 1995. pp. 443–493.
- Baskys A, Gerlai R, Pekhletski R, Roder J, Hampson DR. Physiological and behavioral studies of mice lacking type 4 mGluRs. Neuropharmacology. 1996;35:A2.
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous