Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage - PubMed (original) (raw)
Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage
H Zou et al. Genes Dev. 1997.
Abstract
The bone morphogenetic proteins (BMPs), TGF beta superfamily members, play diverse roles in embryogenesis, but how the BMPs exert their action is unclear and how different BMP receptors (BMPRs) contribute to this process is not known. Here we demonstrate that the two type I BMPRs, BMPR-IA and BMPR-IB, regulate distinct processes during chick limb development. BmpR-IB expression in the embryonic limb prefigures the future cartilage primordium, and its activity is necessary for the initial steps of chondrogenesis. During later chondrogenesis, BmpR-IA is specifically expressed in prehypertrophic chondrocytes. BMPR-IA regulates chondrocyte differentiation, serving as a downstream mediator of Indian Hedgehog (IHH) function in both a local signaling loop and a longer-range relay system to PTHrP. BMPR-IB also regulates apoptosis: Expression of activated BMPR-IB results in increased cell death, and we showed previously that dominant-negative BMPR-IB inhibits apoptosis. Our studies indicate that in TGF beta signaling systems, different type I receptor isoforms are dedicated to specific functions during embryogenesis.
Figures
Figure 4
caBMPR-IA delays chondrocyte differentiation and induces PTHrP expression. Histological sections of day 9 embryonic wings (A) uninfected and (B) caBMPR-IA-infected at stage 14. The infected cartilage element lacked hypertrophic chondrocytes (HC) that were clearly present in the contralateral control. The boxed areas are enlarged on right. (C–F) Serial sections of day 8 uninfected and caBMPR-IA-infected wings (left and right, respectively). Infection was targeted to posterior distal limb mesenchyme at stage 21 resulting in expansion and fusion of the posterior digits. RNA in situ hybridization revealed that (C) Col-II was expressed in both the infected and uninfected limbs, indicating the cells were viable; (D) Col-X was expressed in HC in the uninfected limb and in the proximal part of the infected limb (arrows); however, it could not be detected in the infected digit region; (F) Ihh was expressed in prehypertrophic chondrocytes (preHC) in the uninfected limb and in the proximal part of the infected limb (arrows), but could not be detected in the infected digit region; (E) PTHrP Receptor was expressed in the uninfected chondrocytes (arrows) overlapping the expression domain of Ihh (F) and in the perichondrium; it was also readily detected in the proximal part of the infected limb (arrow), but was not highly localized in the infected digit region. (G) RNA section in situ hybridization of stage 30 tarsal region of uninfected and caBMPR-IA-infected hindlimb (left and right, respectively). PTHrP expression was induced in a broader region of the periarticular cartilage by misexpression of caBMPR-IA. (H) Histological section through an embryonic day 10.3 joint region of a limb infected with caBMPR-IA at stage 14. Ectopic vascularization was scattered throughout the cartilage element, including the joint area (shown here), which normally is not invaded by blood vessels. (I) Schematic drawing of the cartilage differentiation program. Chondrocytes undergo a program of proliferation, maturation from preHC to HC, and, eventually (not shown), calcification and cell death. (J) Model of regulation of chondrocyte differentiation through BMPR-IA-mediated signaling. We propose that there exists a local signal relay loop (depicted by two arrows). Proliferating chondrocytes (expressing PTH/PTHrP Receptor; yellow) exit the cell cycle and begin their maturation from preHC (expressing Ihh and BmpR-IA; red) to HC (expressing Col-X; blue). IHH, produced by preHC, signals to the adjacent perichondrial cells (which express ptc; green; Vortkamp et al. 1996). The perichondrium responds to IHH by expressing Bmps (green) and the BMP proteins signal back to BMPR-IA in the preHC. We also suggest that BMP from the perichondrium is an important relay signal in the proposed regulatory loop between IHH and PTHrP. Perichondrial BMPs could act on (dotted arrow) BMPR-IA in the periarticular region (purple) to regulate PTHrP production. PTHrP then signals to chondrocytes expressing PTH/PTHrP receptor (yellow) to regulate the rate of differentiation. Thus, we suggest that BmpR-IA expression in both the preHC and periarticular chondrocytes is important in regulating the progression of chondrocytes through the differentiation pathway. Model adapted from Vortkamp et al. (1996).
Figure 1
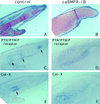
BMPR-IB expression prefigures the future cartilage and its activity is necessary and sufficient for in vivo chondrogenesis. (A–D) Section RNA in situ hybridization with digoxygenin-labeled probes. Stage 27 hindlimb (A) and stage 30 forelimb (C) show that BmpR-IB RNA (purple stain) was strongly expressed in precartilaginous condensation zones, at these stages most strongly in the phalangeal region (arrow). Expression of BmpR-IB preceded that of Col-II (B), an early marker of chondrocytes. BmpR-IB expression decreased in the core region of the cartilage element where Col-II was strongly expressed, but persisted in regions of less mature chondrocytes (arrowheads). (D) BmpR-IA RNA was detected at low levels throughout the limb bud mesenchyme, with highest levels in the distal mesenchyme. (E–G) Day 10 embryos stained with alcian blue to reveal cartilage. (E) Infection of stage 20 forelimb with caBMPR-IB virus or (F) stage 14 presumptive hind-limb field with caBMPR-IA resulted in expanded and fused cartilage elements (right), compared with the contralateral control limb (left). (G) Infection of a stage 21 hindlimb bud with dnBMPR-IB resulted in a loss of distal phalanges (right). Metatarsals were shorter and thinner than in the contralateral limb (left). Regression of the interdigital webbing was inhibited (see also Zou and Niswander 1996). (H,I) Histological section of stage 30 limbs stained with alcian blue. Uninfected left wing has a smooth perichondrium (H). In the contralateral right wing (I) infected at stage 16 with ∼3 times less caBMPR-IB virus than in (E,F), the perichondrium was indistinct and swirls of ectopic mesenchymal condensations were observed in the region of soft tissue (arrowheads). (J,K) BrdU labeling studies. Very few cells incorporated BrdU within the core regions of the cartilage elements of a stage 30 uninfected left wing (J; highlighted by lines), whereas significantly more cells incorporated BrdU throughout the cartilage of the caBMPR-IB-infected contralateral wing (K). High levels of BrdU incorporation in the cartilage were also observed following caBMPR-IA infection (not shown). (L,M) caBMPR-IA results in expansion of the chondrogenic region as distinguished by Col-II expression (L) and induces the ectopic expression of endogenous BmpR-IB (M; arrow). BmpR-IB expression is down-regulated in more mature chondrocytes (arrowheads), similar to normal chondrogenesis.
Figure 2
BMPR-IB activity is necessary for in vitro cartilage formation. High-density micromass cultures derived from stage 22–24 limb buds stained with alcian blue to reveal cartilage nodules after culture for 2 days (top row); 4 days (middle row), or 5 days (bottom row) following (A–C) no viral infection, or infection with (D–F) caBMPR-IB; (G–I) caBMPR-IA; (J–L) dnBMPR-IB; (M–O) dnBMPR-IA virus.
Figure 3
Expression patterns of Bmp, BmpR-IA, and Ihh during cartilage differentiation. (A–C) Serial sections of a stage 31 metatarsal region processed for RNA in situ hybridization with (A) Bmp2, (B) Bmp4, and (C) Bmp7. All three Bmps are expressed in the perichondrium that surrounds the cartilage elements. Serial sections through a stage 32 ulna (D,E) or through the growth plate of an embryonic day 15 distal tibia (F,G) hybridized with (D,F) BmpR-IA or (E,G) Ihh. BmpR-IA and Ihh are largely coexpressed in the prehypertrophic chondrocytes (arrows). BmpR-IA RNA is also detected in the periarticular region, as well as in the inner layer of the perichondrium (arrowheads in F). (H,I) Sections through the periarticular region of a stage 34 carpal region hybridized with (H) BmpR-IA and (I) PTHrP. Both BmpR-IA and PTHrP are expressed in other joints (not shown). Arrows in (A–I) highlight the expression domains.
Figure 5
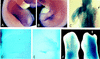
caBMPR-IB does not delay chondrocyte differentiation. Serial sections through the digit region of a day 8.5 uninfected limb (A,C,E) and limb infected with caBMPR-IA at stage 20 (B,D,F). (A,B) Weigert-Safranin stain revealed extensive cartilage formation, fusion of the phalanges, and lack of joint formation in the infected limb. (C–F) RNA section in situ hybridization with PTH/PTHrP receptor (C,D) or Col-X (E,F). In the infected fused phalanges (D,F), PTHrP receptor and Col-X mRNAs displayed complementary expression patterns but were detected along the entire proximodistal axis; in contrast to the discreet proximodistal localization of these RNAs in the contralateral limb (C,E; arrows).
Figure 6
caBMPR-IB regulates cell death in the embryonic limb. (A) Day 7 control uninfected hindlimb. (B) caBMPR-IB infection of the contralateral limb at stage 15 resulted in a very thin limb that also displayed a degenerating AER and distal shortening. (C) In the most dramatic cases, caBMPR-IB infection resulted in extreme truncation of the limb (arrow). (D,E) Nile blue staining to detect nonviable cells. Forty-eight hours after caBMPR-IB infection, the infected forelimb (E) displayed a marked increase in the number of Nile blue-stained mesenchymal and AER cells compared with the contralateral limb (D). (F) Posteriorly localized caBMPR-IB infection of a stage 22 forelimb resulted in an accelerated regression of interdigital soft tissue at stage 30 (right) compared with the contralateral limb (left). The posterior phalanges were expanded and fused.
Figure 7
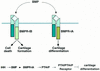
BMPR-specific functions in limb development and molecular pathways elicited by IHH signaling. (Top) We propose that BMPR-IB specifically regulates the intracellular pathways that lead to cell death and formation of the initial cartilaginous skeleton in response to BMP, whereas BMPR-IA regulates the rate of chondrocyte differentiation. Schematic adapted from Massagué and Weis-Garcia (1996). (Bottom) Proposed molecular pathway downstream of IHH. See Discussion for description. Direct interaction is indicated by an arrowhead. Steps indicated by an arrow may be direct or indirect.
Similar articles
- ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development.
Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O'Keefe RJ. Zhang D, et al. J Bone Miner Res. 2003 Sep;18(9):1593-604. doi: 10.1359/jbmr.2003.18.9.1593. J Bone Miner Res. 2003. PMID: 12968668 - BMP signaling stimulates chondrocyte maturation and the expression of Indian hedgehog.
Grimsrud CD, Romano PR, D'Souza M, Puzas JE, Schwarz EM, Reynolds PR, Roiser RN, O'Keefe RJ. Grimsrud CD, et al. J Orthop Res. 2001 Jan;19(1):18-25. doi: 10.1016/S0736-0266(00)00017-6. J Orthop Res. 2001. PMID: 11332615 - Utilization of bone morphogenetic protein receptors during chondrocyte maturation.
Volk SW, D'Angelo M, Diefenderfer D, Leboy PS. Volk SW, et al. J Bone Miner Res. 2000 Aug;15(8):1630-9. doi: 10.1359/jbmr.2000.15.8.1630. J Bone Miner Res. 2000. PMID: 10934663 - Bone morphogenetic proteins.
Chen D, Zhao M, Mundy GR. Chen D, et al. Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review. - The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists.
Nickel J, Dreyer MK, Kirsch T, Sebald W. Nickel J, et al. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S7-14. J Bone Joint Surg Am. 2001. PMID: 11263668 Review.
Cited by
- Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype.
Mabie PC, Mehler MF, Kessler JA. Mabie PC, et al. J Neurosci. 1999 Aug 15;19(16):7077-88. doi: 10.1523/JNEUROSCI.19-16-07077.1999. J Neurosci. 1999. PMID: 10436062 Free PMC article. - Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2.
Dathe K, Kjaer KW, Brehm A, Meinecke P, Nürnberg P, Neto JC, Brunoni D, Tommerup N, Ott CE, Klopocki E, Seemann P, Mundlos S. Dathe K, et al. Am J Hum Genet. 2009 Apr;84(4):483-92. doi: 10.1016/j.ajhg.2009.03.001. Epub 2009 Mar 26. Am J Hum Genet. 2009. PMID: 19327734 Free PMC article. - Parathyroid hormone-related protein: a developmental regulatory molecule necessary for mammary gland development.
Dunbar ME, Wysolmerski JJ. Dunbar ME, et al. J Mammary Gland Biol Neoplasia. 1999 Jan;4(1):21-34. doi: 10.1023/a:1018700502518. J Mammary Gland Biol Neoplasia. 1999. PMID: 10219904 Review. - Metastatic medulloblastoma remodels the local leptomeningeal microenvironment to promote further metastatic colonization and growth.
Abeysundara N, Rasnitsyn A, Fong V, Bahcheli A, Van Ommeren R, Juraschka K, Vladoiu M, Ong W, Livingston B, de Antonellis P, Ly M, Holgado BL, Sirbu O, Bahrampour S, Min HK, Fan J, Nor C, Visvanathan A, Zhang J, Wang H, Qin L, Huang N, Pallotta J, Douglas T, Mak E, Su H, Ng K, Zhang KY, Daniels C, Lucas CG, Eberhart CG, Liu H, Jiang T, Notta F, Ramaswamy V, Reimand J, Gallo M, Rich JN, Wu X, Huang X, Taylor MD. Abeysundara N, et al. Nat Cell Biol. 2025 May;27(5):863-874. doi: 10.1038/s41556-025-01660-7. Epub 2025 Apr 22. Nat Cell Biol. 2025. PMID: 40263572 Free PMC article. - Smurf2 induces degradation of GSK-3beta and upregulates beta-catenin in chondrocytes: a potential mechanism for Smurf2-induced degeneration of articular cartilage.
Wu Q, Huang JH, Sampson ER, Kim KO, Zuscik MJ, O'Keefe RJ, Chen D, Rosier RN. Wu Q, et al. Exp Cell Res. 2009 Aug 15;315(14):2386-98. doi: 10.1016/j.yexcr.2009.05.019. Epub 2009 May 27. Exp Cell Res. 2009. PMID: 19481076 Free PMC article.
References
- Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60:69–82. - PubMed
- Blair SS. Compartments and appendage development in Drosophila. BioEssays. 1995;17:299–309. - PubMed
- Brummel TJ, Twombly V, Marqués G, Wrana JL, Newfeld SJ, O’Connor MB, Gelbart WM. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–261. - PubMed
- Chang SC, Hoang B, Thomas JT, Vukicevi S, Luyten FP, Ryba NJP, Kazak CA, Reddi AH, Moos M. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. - PubMed
- Dewulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for Bone Morphogenetic Proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials