mre11S--a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis - PubMed (original) (raw)
mre11S--a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis
K Nairz et al. Genes Dev. 1997.
Abstract
During meiotic prophase the repair of self-inflicted DNA double-strand break (DSB) damage leads to meiotic recombination in yeast. We employed a genetic screen to specifically characterize cellular functions that become essential after this DSB formation. As a result a new allele of MRE11, termed mre11S (for Separation of functions) was isolated that allows initiation but not processing and repair of meiotic DSBs similar to the well-characterized rad50S allele. In contrast, the mre11-1 allele blocks initiation of meiotic DSBs as reported previously by others. The mre11S allele, which is mutated in the 5' part of the gene, can partially complement mre11 alleles disrupted close to the 3' end that cannot initiate DSBs when homozygous. This suggests homodimerization of the Mre11 protein and the presence of separate domains for DSB initiation and 5' resection. The fact that two genes, RAD50 and MRE11, required for DSB processing are also essential for DSB initiation dictates a model in which a bifunctional initiation/repair complex is required to initiate meiotic recombination. A subset of mre11S nuclei was shown to perform extensive but partially nonhomologous synapsis. We propose that the unprocessed DSBs present in mre11S allow for synapsis, but that homologous synapsis is only ensured at a later stage of recombination.
Figures
Figure 1
Schematic representation of the mutant screen. Three crucial steps of the protocol for the mutant hunt are illustrated. Spores giving rise to red colonies are shaded, and dead spores are marked by a cross.
Figure 2
Alignment of predicted Saccharomyces cerevisiae Mre11 protein sequence with eukaryotic and prokaryotic homologs. Identical amino acids are shaded, S indicates the site of the proline 84 to serine, and I the threonine 188 to isoleucine exchange in mre11S. A potential nuclear localization site is underlined in bold. The first and last amino acids of predicted coiled–coil regions are marked with C. T34, T4, and T10 indicate the approximate transposon insertion sites (see also Fig. 3A).
Figure 3
Intragenic complementation between mre11S and 3′ truncated alleles. (A) Transposon insertion sites at the MRE11 locus. Arrows point in the direction of the lacZ ORF, and the name of the allele is given. Asterisks and S and I indicate the sites of the mre11S mutations. Transposon insertions mre11–T4 and mre11–T10 are in-frame lacZ fusions. (B) Patch assay to study intragenic complementation between mre11S and the various transposon insertion alleles. Complementation is apparent for MRE11/mre11S and to a high extent for heterozygotes involving mre11–T4, mre11–T10, mre11–T40, and mre11–T16. Scarce colonies arising in the mre11S homozygote and other heterozygotes may represent few viable spores or vegetative cells surviving ether treatment.
Figure 4
Meiotic recombination can be induced to an intermediate level in the mre11S homozygote and to clearly higher levels for mre11S/mre11–T4 at ARG4. Recombinants are counted as prototrophs on SC–ARG (SC–HIS) per 10,000 colony-forming units (CFU) on YPD. Data were pooled from two independent experiments. (A,B) Gene conversions at two different meiotic hot spots in homozygous mre11–T4, mre11–T20, and rad50S strains. Mitotic recombination levels can be read at t = 0 and are elevated for mre11–T4 and mre11–T20. Because mitotic recombination is not altered in the rad50S strain, a 10-fold induction during meiosis is apparent. A similar level of meiotic induction for mre11–T4 and mre11–T20 would be masked by the recombinants of mitotic origin. (C,D) Gene conversions at two different meiotic hot spots in MRE11/mre11S, mre11S/mre11S and mre11S/mre11–T4 strains. Normal commitment was observed for MRE11/mre11S, but was reduced ∼50-fold for the homozygous mre11S. The heterozygous mre11S/mre11–T4 was between wild-type and homozygous mre11S at the ARG4 locus but was similar to mre11S at HIS4. (E,F) Decrease of viability [expressed as CFU(t)/CFU(_t_0) × 100 during progression through meiosis of all strains shown in A–D.
Figure 5
Residual resistance to MMS varies over several orders of magnitude for different diploid mre11 mutants. Cells from late exponential phase were plated on YPD containing the indicated amount of MMS and scored after 3 days. Resistance decreased strongly in the strains in the following order except for the first two strains, which were similar: MRE11/MRE11, MRE11/mre11S, mre11S/mre11–T4, mre11S/mre11S, mre11–T4/mre11–T4, mre11–T20/mre11–T20. Y328 (mre11–T20/mre11–T20) did not form visible colonies after 3 days on plates containing 0.005% MMS. The symbol at the end of the dashed line therefore marks the lower limit of detection.
Figure 6
mre11S mutants accumulate meiosis-specific unresected DNA DSBs at recombination hot spots. All experiments shown except for E, were done at the artificial _his4–XLEU2-Mlu_I::_Bam_HI/his4–BLEU2–MluI hot spot and blots were hybridized with probe A (pNKY291). DSB-I and DSB-II mark the location of major cleavage sites. (A) Quantification of the amount of broken DNA at site DSB-I at his4–LEU2 relative to the parental fragment is the result of two independent experiments. The symbols represent the mean value, whereas individual results are symbolized by the error bars. (▪) Y329 (rad50S/rad50S); (□) Y324 (mre11S/mre11–T4); (○) Y323 (mre11S/mre11S). One set of data is C, D, and H. (B) For wild-type strain Y325 (MRE11/mre11S) processed breaks can be seen as a smear at 4 and 5.5 hr. Homozygous rad50S (C) and mre11S (D) strains accumulate unresected DSBs visible as sharp bands in a similar way. (E) Quantification of the amount of broken DNA at the major DSB site close to THR4 relative to the parental fragment (representation analogous to A). (▪) Y329 (rad50S/rad50S); (○) Y323 (mre11S/mre11S); (•) Y292×294 (mre11S rad50S/mre11S rad50S). No DSBs were detectable in strains Y328 (mre11–T20/mre11–T20) (F) and Y327 (mre11–T4/mre11–T4) (G). Asterisks indicate signals corresponding to repetitive DNA on the ethidium bromide-stained gel (not shown; see also Storlazzi et al. 1995). (H) In Y324 (mre11S/mre11–T4) DSB formation was not different from that of mre11S homozygote (D).
Figure 7
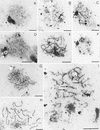
Aberrant SC formation in mre11 mutants shown by electron microscopy. Examples of nuclei classified according to their SC-related morphology are shown. SCs and their precursors are visible as electron dense linear structures, nucleoli (N) as prominent large irregularly shaped dots, and spindle pole bodies (S) as little, round, double spots (bars, 2 μm). (A,B) Class 1 nuclei containing short, unsynapsed axial elements. mre11 nuclei sometimes contain slightly thickened short axial elements as in (B); (C) class 2 nuclei contain both short unsynapsed axial elements and partially synapsed stretches; (D) class 3 nuclei containing elongated unsynapsed axial elements were predominantly observed in mre11S mutants; (E) extensive SC formation (class 4) in a mre11S/mre11S nucleus with only few SC ends visible (the arrow marks a potential partner switch); (F) class 5 nucleus devoid of SC structures possibly past prophase; (G) a rare example of SC formation in mre11–T4 homozygotes; (H) wild-type SCs (class 4) from the MRE11/mre11S strain; (I) partner switches of axial elements confer a network-like appearance to some SCs in the mre11S mutant. Arrows indicate sites of presumed partner switches. A, B, and G are from strain Y327 (mre11–T4/mre11–T4); C–F and I are from Y323 (mre11S/mre11S) and H is from Y325 (MRE11/mre11S).
Figure 8
Kinetics of class 1–5 nuclei during progression through meiotic prophase. Quantitative analysis of SC-related structures of the time course experiment presented in Figs. 6 and 7. Cells past meiosis I are bi- and tetranucleate as observed after DAPI staining. (A–D) Symbols corresponding to class 1–3 and class 5 nuclei and meiotic progression are described in the upper right panel. (E) For clarity, class 4 nuclei exhibiting extensive SCs are presented separately. Wild type exhibited up to 30% SC formation, mre11S mutants up to 7%, but the mre11–T4 homozygote only ∼1%.
Figure 9
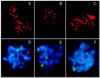
SC formation and homologous pairing are reduced in mre11S. (A–C) Nuclei stained with anti-Zip1 antibody; (D–F) pairing status of chromosome I and IV signals (green and red, respectively). Strains are Y325 (MRE11/mre11S) and Y323 (mre11S/mre11S). (A) Wild-type pachytene SCs; (B) Zip1-positive mre11S nucleus without synapsis; (C) extensive synapsis in mre11S. The upper right patch corresponds to an overexposed Zip1 aggregate. (D) both chromosome I and IV signals are paired in wild type; (E) typical pairing behavior in the mre11S mutant; (F) very rarely both chromosome signals are paired in mre11S.
Figure 10
Model of the relation between DSBs and synapsis. It is proposed that unprocessed DSBs are sufficient to allow for synapsis. Only when DSBs are repaired successfully is homologous synapsis possible. Rare synapsis observed in the mre11–T4 homozygote, where neither induction of meiotic recombination nor DSBs were detected, could be explained if DSBs below the limit of detection were able to induce SC formation. (<) Increasing amount of either SCs or DSBs.
Similar articles
- Interaction of Mre11 and Rad50: two proteins required for DNA repair and meiosis-specific double-strand break formation in Saccharomyces cerevisiae.
Johzuka K, Ogawa H. Johzuka K, et al. Genetics. 1995 Apr;139(4):1521-32. doi: 10.1093/genetics/139.4.1521. Genetics. 1995. PMID: 7789757 Free PMC article. - Functions of the yeast meiotic recombination genes, MRE11 and MRE2.
Ogawa H, Johzuka K, Nakagawa T, Leem SH, Hagihara AH. Ogawa H, et al. Adv Biophys. 1995;31:67-76. doi: 10.1016/0065-227x(95)99383-z. Adv Biophys. 1995. PMID: 7625279 Review. - Coprinus cinereus rad50 mutants reveal an essential structural role for Rad50 in axial element and synaptonemal complex formation, homolog pairing and meiotic recombination.
Acharya SN, Many AM, Schroeder AP, Kennedy FM, Savytskyy OP, Grubb JT, Vincent JA, Friedle EA, Celerin M, Maillet DS, Palmerini HJ, Greischar MA, Moncalian G, Williams RS, Tainer JA, Zolan ME. Acharya SN, et al. Genetics. 2008 Dec;180(4):1889-907. doi: 10.1534/genetics.108.092775. Epub 2008 Oct 20. Genetics. 2008. PMID: 18940790 Free PMC article. - Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination.
Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K. Furuse M, et al. EMBO J. 1998 Nov 2;17(21):6412-25. doi: 10.1093/emboj/17.21.6412. EMBO J. 1998. PMID: 9799249 Free PMC article. - The multiple roles of the Mre11 complex for meiotic recombination.
Borde V. Borde V. Chromosome Res. 2007;15(5):551-63. doi: 10.1007/s10577-007-1147-9. Chromosome Res. 2007. PMID: 17674145 Review.
Cited by
- Processing of DNA double-stranded breaks and intermediates of recombination and repair by Saccharomyces cerevisiae Mre11 and its stimulation by Rad50, Xrs2, and Sae2 proteins.
Ghodke I, Muniyappa K. Ghodke I, et al. J Biol Chem. 2013 Apr 19;288(16):11273-86. doi: 10.1074/jbc.M112.439315. Epub 2013 Feb 26. J Biol Chem. 2013. PMID: 23443654 Free PMC article. - Cancer predisposition and hematopoietic failure in Rad50(S/S) mice.
Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, Oltz EM, Petrini JH. Bender CF, et al. Genes Dev. 2002 Sep 1;16(17):2237-51. doi: 10.1101/gad.1007902. Genes Dev. 2002. PMID: 12208847 Free PMC article. - Mapping meiotic single-strand DNA reveals a new landscape of DNA double-strand breaks in Saccharomyces cerevisiae.
Buhler C, Borde V, Lichten M. Buhler C, et al. PLoS Biol. 2007 Dec;5(12):e324. doi: 10.1371/journal.pbio.0050324. PLoS Biol. 2007. PMID: 18076285 Free PMC article. - Shugoshin promotes sister kinetochore biorientation in Saccharomyces cerevisiae.
Kiburz BM, Amon A, Marston AL. Kiburz BM, et al. Mol Biol Cell. 2008 Mar;19(3):1199-209. doi: 10.1091/mbc.e07-06-0584. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094053 Free PMC article. - The MRE11 complex: starting from the ends.
Stracker TH, Petrini JH. Stracker TH, et al. Nat Rev Mol Cell Biol. 2011 Feb;12(2):90-103. doi: 10.1038/nrm3047. Nat Rev Mol Cell Biol. 2011. PMID: 21252998 Free PMC article. Review.
References
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. - PubMed
- Altshul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Bailey NTJ. Statistical methods in biology. Cambridge, UK: Cambridge University Press; 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous