Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1 - PubMed (original) (raw)
Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1
P Casaccia-Bonnefil et al. Genes Dev. 1997.
Abstract
During development of the central nervous system, oligodendrocyte progenitor cells (O-2A) undergo an orderly pattern of cell proliferation and differentiation, culminating in the ability of oligodendrocytes to myelinate axons. Here we report that p27(Kip1), a cyclin-dependent kinase inhibitor, is an important component of the decision of O-2A cells to withdraw from the cell cycle. In vitro, accumulation of p27 correlates with differentiation of oligodendrocytes. Furthermore, only a fraction of O-2A cells derived from p27-knockout mice differentiate successfully compared to controls. Inability to differentiate correlates with continued proliferation, suggesting that p27 is an important component of the machinery required for the G1/G0 transition in O-2A cells. In vivo, expansion of O-2A precursors before withdrawal, in part, leads to a greater number of oligodendrocytes. Together these data indicate a role for p27 during the decision to withdraw from the cell cycle in the oligodendrocyte lineage.
Figures
Figure 1
Expression of cell cycle proteins in oligodendroglial progenitors and differentiated oligodendrocytes. (A) Changes in the amount of G1 regulatory proteins during oligodendrocyte differentiation. Protein extracts (20–80 μg, depending on the antibody used) obtained from proliferating cortical O-2A precursors (O-2A) or oligodendrocytes maintained in differentiation medium for 5 days (Oligo) were analyzed by Western blot analysis using antibodies indicated on the right of each panel. (B) The protein levels of p27 change during oligodendrocyte differentiation. Protein extracts isolated from O-2A precursors (0) and oligodendrocytes kept in differentiation medium for 1, 3, or 5 days were assayed by Western blot analysis using anti-p27 or anti-CDK-2 antibodies. β-Actin was used as control for protein loading.
Figure 2
Enhanced proliferation of O-2A progenitors in p27 null cultures on day 0. Bright field (a,b) and phase contrast (c,d) photomicrographs of p27+/+ and p27−/− cultures pulsed with 10 μm BrdU for 6 hr on day 0 of plating. Labeled cells were visualized by immunocytochemistry using anti-BrdU antibody. The phase contrast appearance of p27−/− and p27+/+ BrdU-positive cells reveals no difference in cell size. On day 0 of plating, the majority of the cells were round with simple or no processes (c–e). The identity of the cycling cells as proliferating O-2A progenitors was determined by double labeling with BrdU (Texas red) and NG2 (FITC) (f). Bar, 40 μm.
Figure 3
Enhanced proliferation of O-2A progenitors in p27 null cultures after 5 days of mitogen withdrawal. Bright field (a,b) and phase contrast (c,d) photomicrographs of p27+/+ (+/+) and p27−/− (−/−) cells cultured for 5 days in the absence of serum and mitogens. After a 6-hr pulse with 10 μm of BrdU, cells were fixed, stained with antibodies against BrdU, and visualized using diaminobenzidine as substrate. Cycling cells were still observed in the p27−/− cultures (b). Phase contrast reveals that these p27−/− cycling cells have a simple, bipolar morphology (d), whereas the highly branched cells in either p27+/+ (c) or p27−/− (d) cultures do not incorporate BrdU. Bar, 40 μm.
Figure 4
Impaired differentiation of p27−/− oligodendrocyte progenitors. Phase contrast photomicrographs of cultures obtained from p27+/+ (a,c) and p27−/− mice (b,d) maintained in serum-free differentiation medium for 5 days. The majority of p27+/+ precursors differentiate into mature oligodendrocytes, identified by the characteristic highly branched morphology (a,c). In contrast, the majority of p27−/− cultures are characterized by cells with few simple processes (b,d). (a,b) Bar, 30 μm; (c,d) bar, 40 μm.
Figure 5
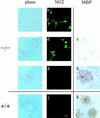
Immunocytochemistry of p27−/− cultures reveals primarily oligodendrocyte progenitors after 5 days in differentiation conditions. Cells from p27−/− (−/−) and p27+/+ (+/+) mice were cultured for 5 days in serum-free differentiation medium and then stained with antibodies against the precursor marker NG2 (b,d,g,j) or the differentiation marker MBP (e,h,k). Phase-contrast photomicrographs are shown only for the cultures stained with anti-NG2 antibodies (a–d,f–g,i–j). The majority of p27−/− cells growing either in isolates (a) or in clusters (c) were oligodendrocyte precursors, as indicated by the simple morphology, NG2 immunoreactivity (b,d), and lack of MBP expression (e). Differentiated oligodendrocytes in the p27−/− (f–h) and in the p27+/+ (i–k) cultures were identified by loss of NG2 immunoreactivity (g,j) and positive MBP immunoreactivity (h,k). Bar, 40 μm.
Figure 6
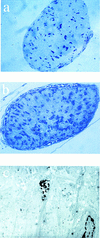
Glial cell number is increased in the optic nerve. Semithin sections (a,b) and electron microscopy of optic nerve from 6-day-old neonatal mice (c). Sections were cut 1 mm anterior of the optic chiasm and stained with toluidine blue and the number of nuclei were counted. Representative sections are shown for p27+/+ (a) and p27−/− mice (b). Electron microscopy of ultrathin sections from p27−/− mice revealed the presence of several apoptotic nuclei. A representative field (c) showing two nuclei (lower right and center of the field), one of which is undergoing apoptosis (center of the field).
Figure 7
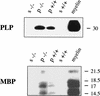
Expression of MBP and PLP are increased in p27−/− mice. Western blot analysis of cytosolic (s, supernatant) and membrane fractions (p, pellet) obtained by differential centrifugation of whole brain homogenates from p27 null (−/−) mice and wild-type (+/+) controls. Equal amount of proteins (100 μg) were loaded and separated by SDS-PAGE. Purified rat myelin (10 μg) was used as a positive control.
Figure 8
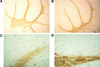
Enhanced MBP immunoreactivity in p27−/− mice. Immunohistochemical analysis of sagittal brain sections from 9-week-old p27+/+ (A,B) and p27−/− (C,D) mice. Increased MBP expression in the cerebellum at low (A,C) and high (B,D) magnification. (A,C) Bar, 300 μm; (B,D) bar, 42.5 μm.
Figure 9
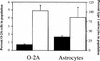
The number of glial cells is increased in p27−/− mice. The bar graph indicates the number of O-2A cells (counted after differential shaking) and type I astrocytes (isolated from the same mixed glial culture), normalized to the number of cells counted immediately after dissection. Solid bars indicate p27+/+; open bars, p27−/− cells.
Figure 10
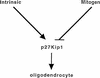
p27 has a central role in differentiation of oligodendrocytes. This model postulates that p27 plays a role in both the intrinsic clock and the mitogen withdrawal differentiation pathway.
References
- Barres B, Lazar M, Raff M. A novel role for thyroid hormone, glucocorticoids, and retinoic acid in timing of oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
- Bögler O, Noble M. Measurement of time in oligodendrocyte-type-2 astrocyte (O-2A) progenitors is a cellular process distinct from differentiation of division. Dev Biol. 1994;162:525–538. - PubMed
- Boulikas T. Phosphorylation of transcription factors and control of the cell cycle. Crit Rev Eukaryot Gene Expr. 1995;5:1–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA68425/CA/NCI NIH HHS/United States
- HD232315/HD/NICHD NIH HHS/United States
- NS21072/NS/NINDS NIH HHS/United States
- R01 NS021072/NS/NINDS NIH HHS/United States
- R01 GM052597/GM/NIGMS NIH HHS/United States
- R56 NS021072/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous