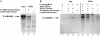Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus - PubMed (original) (raw)
Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus
J R Miller et al. J Cell Biol. 1997.
Abstract
In Xenopus embryos, beta-catenin has been shown to be both necessary and sufficient for the establishment of dorsal cell fates. This signaling activity is thought to depend on the binding of beta-catenin to members of the Lef/Tcf family of transcription factors and the regulation of gene expression by this complex. To test whether beta-catenin must accumulate in nuclei to establish dorsal cell fate, we constructed various localization mutants that restrict beta-catenin to either the plasma membrane, the cytosol, or the nucleus. When overexpressed in Xenopus embryos, the proteins localize as predicted, but surprisingly all forms induce an ectopic axis, indicative of inducing dorsal cell fates. Given this unexpected result, we focused on the membrane-tethered form of beta-catenin to resolve the apparent discrepancy between its membrane localization and the hypothesized role of nuclear beta-catenin in establishing dorsal cell fate. We demonstrate that overexpression of membrane-tethered beta-catenin elevates the level of free endogenous beta-catenin, which subsequently accumulates in nuclei. Consistent with the hypothesis that it is this pool of non-membrane-associated beta-catenin that signals in the presence of membrane-tethered beta-catenin, overexpression of cadherin, which binds free beta-catenin, blocks the axis-inducing activity of membrane- tethered beta-catenin. The mechanism by which ectopic membrane-tethered beta-catenin increases the level of endogenous beta-catenin likely involves competition for the adenomatous polyposis coli (APC) protein, which in other systems has been shown to play a role in degradation of beta-catenin. Consistent with this hypothesis, membrane-tethered beta-catenin coimmunoprecipitates with APC and relocalizes APC to the membrane in cells. Similar results are observed with ectopic plakoglobin, casting doubt on a normal role for plakoglobin in axis specification and indicating that ectopic proteins that interact with APC can artifactually elevate the level of endogenous beta-catenin, likely by interfering with its degradation. These results highlight the difficulty in interpreting the activity of an ectopic protein when it is assayed in a background containing the endogenous protein. We next investigated whether the ability of beta-catenin to interact with potential protein partners in the cell may normally be regulated by phosphorylation. Compared with nonphosphorylated beta-catenin, beta-catenin phosphorylated by glycogen synthase kinase-3 preferentially associates with microsomal fractions expressing the cytoplasmic region of N-cadherin. These results suggest that protein-protein interactions of beta-catenin can be influenced by its state of phosphorylation, in addition to prior evidence that this phosphorylation modulates the stability of beta-catenin.
Figures
Figure 1
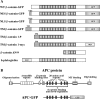
Schematic representation of constructs used in this study. (A) Diagrams depicting the structure of wild-type and localization mutant β-catenin proteins. Some constructs were tagged at the COOH terminus with either GFP (S65T mutant; Heim et al., 1995) or a c-myc epitope (Evan et al., 1985). Shadowed boxes represent the 13 Arm repeats with a nonrepeat sequence between repeats 10 and 11. Sequences directing β-catenin to specific intracellular compartments were added to the NH2 terminus of both wild-type and truncated forms of β-catenin. Wild-type human plakoglobin possesses an overall structure identical to that of β-catenin and is tagged at the NH2 terminus with a c-myc epitope (Merriam et al., 1997). (B) Linear representation of wild-type human APC protein showing conserved motifs, including the oligomerization domain, Arm repeats, 15– and 20–amino acid repeats, microtubule binding domain (MT binding), and discs large binding domain (Dlg binding). The central portion of APC, which is sufficient for β-catenin binding and downregulation (Munemitsu et al., 1995), was tagged at the COOH terminus with GFP (S65T mutant; Heim et al., 1995).
Figure 2
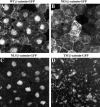
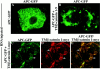
Localization of wild-type and mutant β-catenin–GFP proteins in animal cap cells. The intracellular distribution of each mutant was determined by confocal microscopy (A–D). The WT– β-catenin–GFP protein (A) is localized to the plasma membrane, cytosol, and nucleus in a pattern indistinguishable from that seen for endogenous β-catenin protein (Yost et al., 1996). NES–β-catenin–GFP (B) is present at plasma membrane and at high levels in the cytoplasm. Low levels of fluorescence are detected in the nucleus, which likely reflects the fact that the NES domain does not inhibit nuclear entry but instead promotes the rapid export of tagged proteins from the nucleus. NLS–β-catenin–GFP (C) is predominantly found in the nucleus, and very little fluorescence is observed in the cytoplasm or in association with the plasma membrane. The TM–β-catenin–GFP mutant (D) localizes to intracellular vesicles and organelles in apparent association with the endoplasmic reticulum and Golgi apparatus. TM–β-catenin–GFP fluorescence was never detected in the nucleus.
Figure 3
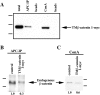
Overexpression of TM–β-catenin results in the stabilization of endogenous β-catenin. Injection of 1.25 ng of control RNA (GFP), TM–β-catenin 1-myc RNA, or TM–β-catenin 1–9 RNA demonstrates overexpression of both forms of TM–β-catenin results in an increase in the steady-state levels of endogenous β-catenin in both total (T) and soluble (S, non–cadherin-bound) lysates. In this experiment, two-cell stage embryos were injected with RNA at four sites, followed by protein extraction at stage 7. To control for protein loading, all endogenous β-catenin bands were normalized to α-spectrin signals from the same Western blot. Numbers below each lane represent the relative level of endogenous β-catenin in each sample (control levels were assigned a value of 1.0). Molecular mass markers indicated are 113 and 75 kD.
Figure 4
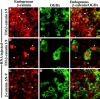
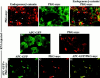
Overexpression of TM–β-catenin results in the accumulation of endogenous β-catenin in the nucleus. RNA encoding TM– β-catenin 1–9 (A–F) was coinjected with Oregon green dextran (OGDx; B, E, and H) as a lineage tracer to determine the effect of TM– β-catenin overexpression on the distribution of endogenous β-catenin (A, C, D, and F). Merged images (C and F) demonstrate that cells overexpressing TM–β-catenin 1–9 possess high levels of endogenous β-catenin in nuclei (arrowheads) in contrast to that observed in nonexpressing cells (arrows). Overexpression of ΔN-9 β-catenin (G–I), that does not promote axis duplication, does not affect the levels of endogenous β-catenin in the nucleus (G and I; arrowheads mark nuclei of cells expressing the ΔN-9 mutant and arrows mark nuclei of nonexpressing cells).
Figure 5
Ectopic TM–β-catenin competes with endogenous β-catenin for binding to endogenous APC and a cadherin fraction. (A) Protein extracted from embryos injected with 1.25 ng of TM–β-catenin 1-myc RNA was subjected to APC immunoprecipitation (APC-IP) or ConA precipitation (ConA, represents cadherin-bound fraction) followed by immunoblotting with anti-myc antibodies. Control lysates were incubated with beads alone (beads). These experiments show that TM–β-catenin 1-myc binds endogenous APC and is present in ConA-bound fractions, indicating an association with cadherin. (B) To determine the effect of overexpression of TM–β-catenin 1-myc on the levels of endogenous β-catenin associated with APC, protein extracts from control embryos or embryos injected with 1.25 ng TM–β-catenin 1-myc were subjected to immunoprecipitation with anti-APC antibodies followed by immunoblotting with anti–β-catenin antibodies. These analyses show that overexpression of TM–β-catenin 1-myc causes a decrease relative to controls in the levels of endogenous β-catenin associated with APC. (Relative levels of endogenous β-catenin are shown below each lane with controls set to 1.0.) (C) Changes in the levels of endogenous β-catenin associated with a cadherin fraction were examined by preparing ConA precipitates from protein extracts prepared from control or TM–β-catenin 1-myc RNA–injected embryos followed by immunoblotting with anti–β-catenin antibodies. The levels of endogenous β-catenin present in ConA fractions decreased to 0.6 of control levels, suggesting that ectopic TM–β-catenin competes with endogenous β-catenin for binding to cadherin. (Relative levels of endogenous β-catenin are shown below each lane.) Molecular mass markers indicated in A are 198, 113, and 75 kD and those indicated in B and C are 113 and 75 kD.
Figure 6
Overexpression of TM–β-catenin results in the redistribution of APC–GFP in animal cap cells. Intracellular distribution of APC–GFP in the absence (A) or presence (B) of TM–β-catenin 1–9. Coexpression of TM–β-catenin 1–9 causes a dramatic redistribution of APC–GFP from a diffuse cytosolic pattern (A) to an apparent association with the secretory apparatus (B). The interaction between TM–β-catenin and APC–GFP was further demonstrated by coexpressing an myc-tagged TM–β-catenin mutant (_TM–β_-catenin 1-myc) with APC–GFP and examining the pattern of each protein in animal cap cells. Both TM–β-catenin 1-myc (C) and APC–GFP (D) proteins display an overlapping intracellular distribution seen in the merged image (E; arrows in C–E).
Figure 7
Overexpression of plakoglobin causes the accumulation of endogenous β-catenin in the nucleus and the redistribution of APC–GFP. Human plakoglobin was overexpressed in animal cap cells, and the distribution of both endogenous β-catenin (A and C) and myc-tagged plakoglobin (B and C) was determined by confocal microscopy. Cells expressing ectopic plakoglobin possess high levels of endogenous β-catenin in the nucleus (arrowheads in A and C) when compared to cells not expressing ectopic plakoglobin (arrows in A and C). Overexpression of plakoglobin also results in the redistribution of APC–GFP within the cell to sites of plakoglobin accumulation. The localization of APC–GFP (D) and ectopic plakoglobin (E) in the absence of other RNAs demonstrates the different localization patterns of the two ectopic proteins. Coinjecting APC–GFP and plakoglobin, however, results in the redistribution of APC–GFP (F) to a pattern indistinguishable from that seen for ectopic plakoglobin (G). Overlapping APC–GFP (F) and plakoglobin (G) staining appears as yellow staining in the merged image (H). Arrows mark examples of APC–GFP and plakoglobin colocalization.
Figure 8
Phosphorylation of β-catenin by Xgsk-3 enhances association with N-cadherin in vitro. (A) β-catenin was translated in vitro in the presence of [35S]methionine and, in separate reactions, microsomal membranes were incubated in rabbit reticulocyte lysate with unlabeled methionine in the presence or absence of N-cadherin RNA. The β-catenin reactions and microsome reactions were then mixed, and the samples were incubated to allow binding of β-catenin to the microsomal membranes. The samples were then centrifuged to generate microsomal pellets and supernatants. Lane 1, 35S–β-catenin present in the supernatant after centrifugation. Adjusting for volumes, these lanes contain 40% of the total supernatant. Lane 2, 35S–β-catenin associated with the pelleted microsomes containing N-cadherin. Lane 3, 35S–β-catenin associated with control microsomes. The data show that a small percentage of the total 35S-labeled β-catenin binds microsomal membranes in a cadherin-dependent manner (compare lanes 1 and 2). (B) β-catenin and Xgsk-3 RNAs, as well as microsomal membranes with or without N-cadherin RNAs, were translated in vitro with nonradioactive methionine and then incubated with or without recombinant Xgsk-3 protein in the presence of [γ32P]ATP. After centrifugation, the samples were immunoprecipitated with an anti–β-catenin antibody. Lanes 1 and 5, immunoprecipitation of β-catenin from the supernatant (lane 1) and microsome/N-cadherin pellet (lane 5) after cotranslation with Xgsk-3 RNA demonstrates that most of the phosphorylated β-catenin associates with the microsomal pellet. Lanes 2 and 6, immunoprecipitation of β-catenin from the supernatant (lane 2) and pellet (lane 6) of β-catenin translations incubated with recombinant Xgsk-3 protein confirms the conclusion of lanes 1 and 5. Lanes 3 and 7, omission of N-cadherin RNA demonstrates that most phosphorylated β-catenin is immunoprecipitated from the supernatant (lane 3) and not the microsomal pellet lacking N-cadherin (lane 7). Lanes 4 and 8, omission of exogenous Xgsk-3 protein and RNA demonstrates basal phosphorylation of β-catenin by kinases present in the lysate, that is substantially lower than in samples supplemented with Xgsk-3, and this phosphorylated β-catenin accumulates primarily in the supernatant (lane 4) rather than the pellet (lane 8), even though the microsomes contained N-cadherin.
Figure 9
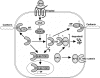
Model of the Wnt signaling pathway showing competition between endogenous and ectopic β-catenin for interactions with several protein partners, including cadherin, APC, and Lef/ Tcf. Overexpression of various β-catenin mutants that possess the ability to interact with APC (TM–β-catenin is shown) is predicted to increase the stability of endogenous β-catenin. The accumulation of endogenous β-catenin via activation of the Wnt pathway or expression of ectopic β-catenin both result in the increase of “free” β-catenin capable of interacting with members of the Lef/Tcf family of transcription factors. This β-catenin transcription factor complex translocates into the nucleus, where it regulates the expression of target genes responsible for establishing dorsal cell fate. Furthermore, phosphorylation of β-catenin by XGSK-3 may not only regulate β-catenin stability but may also result in competition between phosphorylated and nonphosphorylated β-catenin isoforms for binding to the cytoplasmic domain of cadherin.
Similar articles
- Lef/Tcf-dependent Wnt/beta-catenin signaling during Xenopus axis specification.
Geng X, Xiao L, Lin GF, Hu R, Wang JH, Rupp RA, Ding X. Geng X, et al. FEBS Lett. 2003 Jul 17;547(1-3):1-6. doi: 10.1016/s0014-5793(03)00639-2. FEBS Lett. 2003. PMID: 12860376 - Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling.
Klymkowsky MW, Williams BO, Barish GD, Varmus HE, Vourgourakis YE. Klymkowsky MW, et al. Mol Biol Cell. 1999 Oct;10(10):3151-69. doi: 10.1091/mbc.10.10.3151. Mol Biol Cell. 1999. PMID: 10512857 Free PMC article. - Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification.
Farr GH 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Farr GH 3rd, et al. J Cell Biol. 2000 Feb 21;148(4):691-702. doi: 10.1083/jcb.148.4.691. J Cell Biol. 2000. PMID: 10684251 Free PMC article. - TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling.
Brantjes H, Barker N, van Es J, Clevers H. Brantjes H, et al. Biol Chem. 2002 Feb;383(2):255-61. doi: 10.1515/BC.2002.027. Biol Chem. 2002. PMID: 11934263 Review. - New steps in the Wnt/beta-catenin signal transduction pathway.
Sakanaka C, Sun TQ, Williams LT. Sakanaka C, et al. Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
- Wnt11b is involved in cilia-mediated symmetry breakage during Xenopus left-right development.
Walentek P, Schneider I, Schweickert A, Blum M. Walentek P, et al. PLoS One. 2013 Sep 13;8(9):e73646. doi: 10.1371/journal.pone.0073646. eCollection 2013. PLoS One. 2013. PMID: 24058481 Free PMC article. - ATP4a is required for development and function of the Xenopus mucociliary epidermis - a potential model to study proton pump inhibitor-associated pneumonia.
Walentek P, Beyer T, Hagenlocher C, Müller C, Feistel K, Schweickert A, Harland RM, Blum M. Walentek P, et al. Dev Biol. 2015 Dec 15;408(2):292-304. doi: 10.1016/j.ydbio.2015.03.013. Epub 2015 Apr 4. Dev Biol. 2015. PMID: 25848696 Free PMC article. - Requirement for beta-catenin in anterior-posterior axis formation in mice.
Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Huelsken J, et al. J Cell Biol. 2000 Feb 7;148(3):567-78. doi: 10.1083/jcb.148.3.567. J Cell Biol. 2000. PMID: 10662781 Free PMC article. - Requirement for a nuclear function of beta-catenin in Wnt signaling.
Cong F, Schweizer L, Chamorro M, Varmus H. Cong F, et al. Mol Cell Biol. 2003 Dec;23(23):8462-70. doi: 10.1128/MCB.23.23.8462-8470.2003. Mol Cell Biol. 2003. PMID: 14612392 Free PMC article. - A role for cyclin-dependent kinase(s) in the modulation of fast anterograde axonal transport: effects defined by olomoucine and the APC tumor suppressor protein.
Ratner N, Bloom GS, Brady ST. Ratner N, et al. J Neurosci. 1998 Oct 1;18(19):7717-26. doi: 10.1523/JNEUROSCI.18-19-07717.1998. J Neurosci. 1998. PMID: 9742142 Free PMC article.
References
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. - PubMed
- Brannon M, Kimelman D. Activation of Siamoisby the Wnt pathway. Dev Biol. 1996;180:344–347. - PubMed
- Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. . Nature (Lond) 1997;385:829–833. - PubMed
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development (Camb) 1996;122:3055–3065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous