Oxidative damage during aging targets mitochondrial aconitase - PubMed (original) (raw)
Oxidative damage during aging targets mitochondrial aconitase
L J Yan et al. Proc Natl Acad Sci U S A. 1997.
Erratum in
- Proc Natl Acad Sci U S A 1998 Feb 17;95(4):1968
Abstract
The mechanisms that cause aging are not well understood. The oxidative stress hypothesis proposes that the changes associated with aging are a consequence of random oxidative damage to biomolecules. We hypothesized that oxidation of specific proteins is critical in controlling the rate of the aging process. Utilizing an immunochemical probe for oxidatively modified proteins, we show that mitochondrial aconitase, an enzyme in the citric acid cycle, is a specific target during aging of the housefly. The oxidative damage detected immunochemically was paralleled by a loss of catalytic activity of aconitase, an enzyme activity that is critical in energy metabolism. Experimental manipulations which decrease aconitase activity should therefore cause a decrease in life-span. This expected decrease was observed when flies were exposed to hyperoxia, which oxidizes aconitase, and when they were given fluoroacetate, an inhibitor of aconitase. The identification of a specific target of oxidative damage during aging allows for the assessment of the physiological age of a specific individual and provides a method for the evaluation of treatments designed to affect the aging process.
Figures
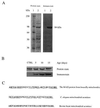
Figure 1
Identification of housefly mitochondrial aconitase as a target of oxidative damage. (A) Immunochemical detection of protein carbonyls in mitochondria from flight muscles of houseflies (15 days old). DNPH-treated mitochondrial matrix proteins were electrophoresed on SDS/PAGE (8.5% resolving gel) and transferred to Immobilon-P membrane. Oxidized proteins were detected immunochemically as described in the text. Lane 1 shows protein standard markers, and lane 2 contains mitochondrial matrix proteins. Protein was stained with Coomassie blue. An 84-kDa protein in the matrix exhibited a strong immunoreaction for the carbonyl groups. (B) Increase in carbonyl content of the 84-kDa protein in housefly at different ages. (Upper) The protein band stained with amido black. (Lower) The immunostain. The control (CTRL) shown is 5-day-old fly mitochondrial matrix protein without DNPH treatment. A 25-μg protein was applied onto SDS/PAGE in both A and B. (C) A computer-assisted search from protein database identified the 84-kDa protein as mitochondrial aconitase. The underlined amino acids show the N-terminal amino acid sequence homology in mitochondrial aconitase from the housefly and Caenorhabditis elegans; whereas the amino acids in bold show the homology with bovine heart mitochondrial aconitase.
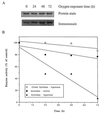
Figure 2
(A) Mitochondrial aconitase activity in the flight muscles of the houseflies at different ages. Two different methods were used to determine aconitase activity. Method 1 (▪) is a coupled assay in which the formation of NADPH was followed at 340 nm, using isocitrate as a substrate (23). Method 2 (⧫) is based on the formation of isocitrate from cis-aconitate, measured as the decrease in absorbance at 240 nm (24). (B) Immunochemical quantitation of aconitase carbonyls in 15-day-old flyers and crawlers, collected from the same population of flies. The procedure was the same as in Fig. 1_A_. (C) Aconitase enzyme activity in mitochondria from the flight muscles of 15-day-old flyers and crawlers, collected from the same population of flies. Enzyme activity was determined by method 1.
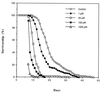
Figure 3
(A) Immunochemical detection of aconitase carbonyls in houseflies exposed to 100% ambient oxygen. The assay was performed as described in Fig. 1. (B) Effect of 100% ambient oxygen on the activities of mitochondrial aconitase and citrate synthase. Six-day-old flies were exposed to 100% oxygen for indicated times. Aconitase activity was determined by method 1, as described in the legend of Fig. 2. Citrate synthase was assayed according to Srere (25).
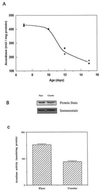
Figure 4
Survivorship curves of adult male houseflies administered with different concentrations of fluoroacetate in their drinking water. Flies were housed in 1-ft3 cages with 200 flies per cage. A concentration of 1 mM resulted in 80% mortality within 24 h. Data are based on the mortality of 200 flies in each population.
Similar articles
- Selectivity of protein oxidative damage during aging in Drosophila melanogaster.
Das N, Levine RL, Orr WC, Sohal RS. Das N, et al. Biochem J. 2001 Nov 15;360(Pt 1):209-16. doi: 10.1042/0264-6021:3600209. Biochem J. 2001. PMID: 11696009 Free PMC article. - In the aging housefly aconitase is the only citric acid cycle enzyme to decline significantly.
Yarian CS, Sohal RS. Yarian CS, et al. J Bioenerg Biomembr. 2005 Apr;37(2):91-6. doi: 10.1007/s10863-005-4132-z. J Bioenerg Biomembr. 2005. PMID: 15906154 - Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species.
Lushchak OV, Piroddi M, Galli F, Lushchak VI. Lushchak OV, et al. Redox Rep. 2014 Jan;19(1):8-15. doi: 10.1179/1351000213Y.0000000073. Epub 2013 Nov 22. Redox Rep. 2014. PMID: 24266943 Free PMC article. Review. - Aconitases: Non-redox Iron-Sulfur Proteins Sensitive to Reactive Species.
Castro L, Tórtora V, Mansilla S, Radi R. Castro L, et al. Acc Chem Res. 2019 Sep 17;52(9):2609-2619. doi: 10.1021/acs.accounts.9b00150. Epub 2019 Jul 9. Acc Chem Res. 2019. PMID: 31287291 Review.
Cited by
- Impact of singular versus combinatorial environmental stress on RONS generation in Drosophila melanogaster larvae.
Bomble P, Nath BB. Bomble P, et al. Front Physiol. 2024 Aug 29;15:1426169. doi: 10.3389/fphys.2024.1426169. eCollection 2024. Front Physiol. 2024. PMID: 39318365 Free PMC article. - Neuroprotective treatment with the nitrone compound OKN-007 mitigates age-related muscle weakness in aging mice.
Xu H, Piekarz KM, Brown JL, Bhaskaran S, Smith N, Towner RA, Van Remmen H. Xu H, et al. Geroscience. 2024 Oct;46(5):4263-4273. doi: 10.1007/s11357-024-01134-y. Epub 2024 Mar 21. Geroscience. 2024. PMID: 38512579 Free PMC article. - Implications of metabolism on multi-systems healthy aging across the lifespan.
Yao S, Colangelo LA, Perry AS, Marron MM, Yaffe K, Sedaghat S, Lima JAC, Tian Q, Clish CB, Newman AB, Shah RV, Murthy VL. Yao S, et al. Aging Cell. 2024 Apr;23(4):e14090. doi: 10.1111/acel.14090. Epub 2024 Jan 29. Aging Cell. 2024. PMID: 38287525 Free PMC article. - Analysis of oxygen consumption rates in zebrafish reveals differences based on sex, age and physical activity recovery.
Konadu B, Hosler JP, Gibert Y, Edwards KS. Konadu B, et al. Front Physiol. 2023 Sep 13;14:1272366. doi: 10.3389/fphys.2023.1272366. eCollection 2023. Front Physiol. 2023. PMID: 37781232 Free PMC article. - Mitochondrial aconitase suppresses immunity by modulating oxaloacetate and the mitochondrial unfolded protein response.
Kim E, Annibal A, Lee Y, Park HH, Ham S, Jeong DE, Kim Y, Park S, Kwon S, Jung Y, Park J, Kim SS, Antebi A, Lee SV. Kim E, et al. Nat Commun. 2023 Jun 22;14(1):3716. doi: 10.1038/s41467-023-39393-6. Nat Commun. 2023. PMID: 37349299 Free PMC article.
References
- Harman D. J Gerontol. 1956;11:298–300. - PubMed
- Sheldahl J A, Tappel A L. Exp Gerontol. 1974;9:33–41. - PubMed
- Stadtman E R. Science. 1992;257:1220–1224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources