Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity - PubMed (original) (raw)
Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity
J Lingner et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Telomerase is a specialized reverse transcriptase consisting of both RNA and protein components. Previous characterization of yeast telomerase function in vivo identified four EST (for ever shorter telomeres) genes that, when mutated, result in the phenotypes expected for a defect in telomerase. Consistent with this genetic prediction, the EST2 gene has recently been shown to encode the catalytic component of telomerase. Using an in vitro assay, we show here that telomerase activity is present in extracts prepared from yeast strains carrying est1-Delta, est3-Delta, and cdc13-2(est) mutations. Therefore, while these three genes are necessary for telomerase function in vivo, they do not encode components essential for core catalytic activity. When Est2p, the one EST gene product found to be essential for catalytic activity, was immunoprecipitated from extracts, the telomerase RNA subunit was also specifically precipitated, supporting the conclusion that these two components are in a stable complex.
Figures
Figure 1
A quadruple-mutant _est1_-Δ _est2_-Δ _est3_-Δ cdc13–2 est strain has the same phenotype as single-mutant est strains. Haploid quadruple- and single-mutant strains were generated by dissection of DVL176, and freshly germinated haploid spores of the indicated genotype were streaked for single colonies on YEPD (yeast extract/peptone/dextrose) rich medium plates. Two successive streak-outs, differing from each other by ≈25 generations (g.), were assembled on YEPD plates and incubated for 3 days at 30°C prior to photography. A total of four to five strains for each genotype were analyzed in parallel, with similar results observed for each genotype; a representative example is shown for each.
Figure 2
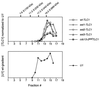
Sedimentation of the yeast telomerase RNP from est mutant strains. Yeast extracts were fractionated by glycerol gradient sedimentation, and the yeast TLC1 RNA (Upper) and U1 snRNA (Lower, shown for the EST+ strain) were quantitated by PhosphorImager analysis following Northern blotting. The U1 snRNA distribution was consistently bimodal, which may represent the free snRNP and higher-order complexes. Marker proteins were run in a parallel gradient (alcohol dehydrogenase, 7.6S; catalase, 11.3S; apoferritin, 17.3S; and thyroglobulin, 19.3S).
Figure 3
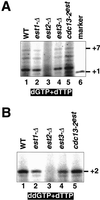
Telomerase activity is present in _est1_-Δ, _est3_-Δ, and cdc13–2 est strains. (A) Telomerase activity as measured in the standard reaction, in the presence of [α-32P]dTTP and dGTP. Pooled glycerol gradient fractions were concentrated, to equalize the amount of telomerase RNA in the various samples. The +1 marker was generated by terminal deoxynucleotidyltransferase extension of the same primer used in the telomerase assays with [α-33P]ddTTP. (B) Enzyme activity monitored in the presence of [α-32P]dTTP and the chain-terminator ddGTP, using the same pooled fractions as in A.
Figure 4
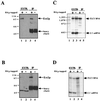
Est2p is in a complex with the yeast telomerase RNA. Immunoprecipitation of Est2p and TLC1 RNA from extracts of a HA3-tagged Est2 strain and of a control untagged Est2 strain by incubation with the anti-HA antibody. (A) Western blot, using 12CA5 antibody; lanes 1 and 2, 5 μl (35 μg) of extracts from strains carrying pVL782 (HA3-tagged Est2) or pVL369 (untagged Est2), respectively; lanes 3 and 4, immunoprecipitates derived from 550 μl of the same extracts used in lanes 1 and 2, respectively. (B) Western blot as in A; lanes 1 and 2, 10 μl (60 μg) of extracts from strains carrying pVL782 (HA3-tagged Est2) or pVL406 (untagged Est2), respectively; lanes 3 and 4, immunoprecipitates derived from 250 μl of the same extracts used in lanes 1 and 2, respectively. A longer exposure is shown for lanes 1 and 2 than for lanes 3 and 4; the ≈50-kDa band in lanes 1 and 2 is a protein that crossreacts with the 12CA5 antibody, detected on long exposures. (C) Northern blot, using TLC1 and U1 probes; lane 1, molecular mass markers; lanes 2 and 3, RNA extracted from the same extracts and extract volume as in lanes 1 and 2, part A; lanes 4 and 5, RNA extracted from the same immunoprecipitates (and same volume) as shown in lanes 3 and 4, part A. (D) Northern blot, using TLC1 and U1 probes; lanes 1 and 2, RNA extracted from the same extracts and extract volume as in lanes 1 and 2, part B; lanes 3 and 4, RNA extracted from the same immunoprecipitates (and same volume) as shown in lanes 3 and 4, part B.
Similar articles
- The Est3 protein is a subunit of yeast telomerase.
Hughes TR, Evans SK, Weilbaecher RG, Lundblad V. Hughes TR, et al. Curr Biol. 2000 Jun 29;10(13):809-12. doi: 10.1016/s0960-9822(00)00562-5. Curr Biol. 2000. PMID: 10898986 - Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes.
Lendvay TS, Morris DK, Sah J, Balasubramanian B, Lundblad V. Lendvay TS, et al. Genetics. 1996 Dec;144(4):1399-412. doi: 10.1093/genetics/144.4.1399. Genetics. 1996. PMID: 8978029 Free PMC article. - Est1 and Cdc13 as comediators of telomerase access.
Evans SK, Lundblad V. Evans SK, et al. Science. 1999 Oct 1;286(5437):117-20. doi: 10.1126/science.286.5437.117. Science. 1999. PMID: 10506558 - Telomerase: what are the Est proteins doing?
Taggart AK, Zakian VA. Taggart AK, et al. Curr Opin Cell Biol. 2003 Jun;15(3):275-80. doi: 10.1016/s0955-0674(03)00040-1. Curr Opin Cell Biol. 2003. PMID: 12787768 Review. - The role of the EST genes in yeast telomere replication.
Hughes TR, Morris DK, Salinger A, Walcott N, Nugent CI, Lundblad V. Hughes TR, et al. Ciba Found Symp. 1997;211:41-7; discussion 47-52, 71-5. doi: 10.1002/9780470515433.ch4. Ciba Found Symp. 1997. PMID: 9524750 Review.
Cited by
- Active Yeast Telomerase Shares Subunits with Ribonucleoproteins RNase P and RNase MRP.
Lemieux B, Laterreur N, Perederina A, Noël JF, Dubois ML, Krasilnikov AS, Wellinger RJ. Lemieux B, et al. Cell. 2016 May 19;165(5):1171-1181. doi: 10.1016/j.cell.2016.04.018. Epub 2016 May 5. Cell. 2016. PMID: 27156450 Free PMC article. - Modulation of yeast telomerase activity by Cdc13 and Est1 in vitro.
Chen YF, Lu CY, Lin YC, Yu TY, Chang CP, Li JR, Li HW, Lin JJ. Chen YF, et al. Sci Rep. 2016 Sep 23;6:34104. doi: 10.1038/srep34104. Sci Rep. 2016. PMID: 27659693 Free PMC article. - The anaphase promoting complex contributes to the degradation of the S. cerevisiae telomerase recruitment subunit Est1p.
Ferguson JL, Chao WC, Lee E, Friedman KL. Ferguson JL, et al. PLoS One. 2013;8(1):e55055. doi: 10.1371/journal.pone.0055055. Epub 2013 Jan 25. PLoS One. 2013. PMID: 23372810 Free PMC article. - Deletion of the telomerase reverse transcriptase gene and haploinsufficiency of telomere maintenance in Cri du chat syndrome.
Zhang A, Zheng C, Hou M, Lindvall C, Li KJ, Erlandsson F, Björkholm M, Gruber A, Blennow E, Xu D. Zhang A, et al. Am J Hum Genet. 2003 Apr;72(4):940-8. doi: 10.1086/374565. Epub 2003 Mar 10. Am J Hum Genet. 2003. PMID: 12629597 Free PMC article. - Loss of Ku's DNA end binding activity affects telomere length via destabilizing telomere-bound Est1 rather than altering TLC1 homeostasis.
Lemon LD, Morris DK, Bertuch AA. Lemon LD, et al. Sci Rep. 2019 Jul 23;9(1):10607. doi: 10.1038/s41598-019-46840-2. Sci Rep. 2019. PMID: 31337791 Free PMC article.
References
- Blackburn E H, Greider C W, editors. Telomeres. Plainview, NY: Cold Spring Harbor Lab. Press; 1995.
- Greider C W, Blackburn E H. Nature (London) 1989;337:331–337. - PubMed
- Shippen-Lentz D E, Blackburn E H. Science. 1990;247:546–552. - PubMed
- Romero D P, Blackburn E H. Cell. 1991;67:343–353. - PubMed
- Lingner J, Hendrick L L, Cech T R. Genes Dev. 1994;8:1984–1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials