MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death - PubMed (original) (raw)
MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death
D K Han et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Activation of the cascade of proteolytic caspases has been identified as the final common pathway of apoptosis in diverse biological systems. We have isolated a gene, termed MRIT, that possesses overall sequence homology to FLICE (MACH), a large prodomain caspase that links the aggregated complex of the death domain receptors of the tumor necrosis factor receptor family to downstream caspases. However, unlike FLICE, the C-terminal domain of MRIT lacks the caspase catalytic consensus sequence QAC(R/Q)G. Nonetheless MRIT activates caspase-dependent death. Using yeast two-hybrid assays, we demonstrate that MRIT associates with caspases possessing large and small prodomains (FLICE, and CPP32/YAMA), as well as with the adaptor molecule FADD. In addition, MRIT simultaneously and independently interacts with BclXL and FLICE in mammalian cells. Thus, MRIT is a mammalian protein that interacts simultaneously with both caspases and a Bcl-2 family member.
Figures
Figure 1
(On the opposite page.) Sequence characterization of MRIT isoforms. (A) Deduced amino acid sequence of naturally occurring MRIT isoforms. (B) Alignment of MRIT DEDs with known DED-containing proteins. Similar to FLICE, MRIT also has two DEDs (10, 18). Black residues indicate sequence identity and conserved residues are indicated by shaded residues. (C) Alignment of ICE-homology domain of MRIT with FLICE/MACH1 (residues 237–479), YAMA/CPP32 (residues 39–277), ICE-LAP6 (residues 163–416), and ICE-LAP3 (residues 70–303). (D) Diagrammatic representation of MRIT isoforms and deletion mutants. Black boxes in the C termini of MRITα2 and MRITβ1 represent unique 26 and 19 residues, respectively. Gray boxes represent the DED-1 and DED-2.
Figure 2
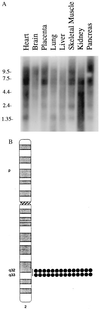
(A) Northern blot analysis of poly(A)+ RNA using a full-length cDNA probe reveals that multiple transcripts of MRIT are expressed in human adult tissues. (B) A 1.2-kb EST clone (IMAGE consortium clone 26915) was used to hybridized (at 50 ng/μl) to metaphase cells prepared from phytohemagglutinin-stimulated, BrdU-pulsed, normal male lymphocytes. Fluorescent signals (indicated by dots) were mapped to each of 16 metaphases (signals were seen at 2q32–33 on 28 of 32 homologs). The line indicates the range of locations observed.
Figure 3
MRIT binds to large and small prodomain caspases (FLICE, CPP32/YAMA) in vitro. Recombinant His-tagged MRITα1 or -β1 immobilized on Ni2+ beads were incubated with in vitro translated 35S-labeled FLICE, CPP32/YAMA, or with CrmA. After extensive washing, the bound-labeled proteins were separated by SDS/PAGE and analyzed by autoradiography. The amount of corresponding input radiolabeled protein is shown A. CrmA serves as a negative control for nonspecific binding.
Figure 4
Direct association of MRIT with FLICE and BclXL in mammalian cells. MRITα1 can directly associates with FLICE–DED–AU1 (A) or FLICE–AU1 (B) and BclXL. BHK-CrmA cells were transiently transfected with indicated expression constructs. Thirty-six hours after transfection, extracts were prepared and immunoprecipitated (IP) with mAb to AU1-l coupled to Sepharose beads (Babco, Richmond, CA), mAb to FLAG coupled to agarose beads (Kodak), or with His beads (Invitrogen). Samples were then blotted with mAb to AU1, FLAG, or 6-His (Babco) and Antiexpress (Invitrogen).
Figure 5
MRIT induces cell death in BHK cells. (A) Cell death mediation by MRITα1 and protection by cotransfection with CrmA (B). (C) Quantitation of cell death induced by MRIT isoforms. BHK cells were cotransfected with indicated MRIT isoforms (1 μg) along with a β-galactosidase expression construct (0.3 μg) using Superfect (Qiagen, Chatsworth, CA). Positive cells showing β-galactosidase activity and apoptotic (round and condensed) morphology were scored after 36–48 hr. Representative results from at least five independent experiment is shown. (D) Overexpression of MRIT (0.5 μg) in BHK cells induces apoptosis that is blocked by CrmA (0.5 μg) and z-VAD-fmk (10 μM). (E) Cell death mediated by overexpression of MRITα1 (0.1 μg) in MCF7 cells is also blocked by Bcl-2 (0.5 μg) and BclXL (0.5 μg).
Similar articles
- Casper is a FADD- and caspase-related inducer of apoptosis.
Shu HB, Halpin DR, Goeddel DV. Shu HB, et al. Immunity. 1997 Jun;6(6):751-63. doi: 10.1016/s1074-7613(00)80450-1. Immunity. 1997. PMID: 9208847 - CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis.
Inohara N, Koseki T, Hu Y, Chen S, Núñez G. Inohara N, et al. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10717-22. doi: 10.1073/pnas.94.20.10717. Proc Natl Acad Sci U S A. 1997. PMID: 9380701 Free PMC article. - Identification and characterization of DEDD2, a death effector domain-containing protein.
Roth W, Stenner-Liewen F, Pawlowski K, Godzik A, Reed JC. Roth W, et al. J Biol Chem. 2002 Mar 1;277(9):7501-8. doi: 10.1074/jbc.M110749200. Epub 2001 Dec 11. J Biol Chem. 2002. PMID: 11741985 - FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis.
Krueger A, Baumann S, Krammer PH, Kirchhoff S. Krueger A, et al. Mol Cell Biol. 2001 Dec;21(24):8247-54. doi: 10.1128/MCB.21.24.8247-8254.2001. Mol Cell Biol. 2001. PMID: 11713262 Free PMC article. Review. No abstract available. - Caspases: the executioners of apoptosis.
Cohen GM. Cohen GM. Biochem J. 1997 Aug 15;326 ( Pt 1)(Pt 1):1-16. doi: 10.1042/bj3260001. Biochem J. 1997. PMID: 9337844 Free PMC article. Review.
Cited by
- Regulated necrosis, a proinflammatory cell death, potentially counteracts pathogenic infections.
Zhang G, Wang J, Zhao Z, Xin T, Fan X, Shen Q, Raheem A, Lee CR, Jiang H, Ding J. Zhang G, et al. Cell Death Dis. 2022 Jul 22;13(7):637. doi: 10.1038/s41419-022-05066-3. Cell Death Dis. 2022. PMID: 35869043 Free PMC article. Review. - Cell death pathways: intricate connections and disease implications.
Kist M, Vucic D. Kist M, et al. EMBO J. 2021 Mar 1;40(5):e106700. doi: 10.15252/embj.2020106700. Epub 2021 Jan 13. EMBO J. 2021. PMID: 33439509 Free PMC article. Review. - Multiple roles of caspase-8 in cell death, inflammation, and innate immunity.
Orning P, Lien E. Orning P, et al. J Leukoc Biol. 2021 Jan;109(1):121-141. doi: 10.1002/JLB.3MR0420-305R. Epub 2020 Jun 12. J Leukoc Biol. 2021. PMID: 32531842 Free PMC article. Review. - Conservation of structure and function in vertebrate c-FLIP proteins despite rapid evolutionary change.
Sakamaki K, Iwabe N, Iwata H, Imai K, Takagi C, Chiba K, Shukunami C, Tomii K, Ueno N. Sakamaki K, et al. Biochem Biophys Rep. 2015 Aug 7;3:175-189. doi: 10.1016/j.bbrep.2015.08.005. eCollection 2015 Sep. Biochem Biophys Rep. 2015. PMID: 29124180 Free PMC article.
References
- Alnemri E S, Livingston D J, Nichloson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Cell. 1997;87:171. - PubMed
- Miller D K. Semin Immunol. 1997;9:35–49. - PubMed
- Yuan J, Shaham S, Ledoux S, Ellis H, M, Horvitz H R. Cell. 1993;75:641–652. - PubMed
- Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mankovich J, Shi L, Greenberg A H, Miller L K, Wong W W. Science. 1995;269:1885–1888. - PubMed
- Hengartner M O, Horvitz H R. Cell. 1994;76:665–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM037905/GM/NIGMS NIH HHS/United States
- P51 RR000166/RR/NCRR NIH HHS/United States
- P01 HL003174/HL/NHLBI NIH HHS/United States
- GM37905/GM/NIGMS NIH HHS/United States
- RR00166/RR/NCRR NIH HHS/United States
- HL03175/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials