Translational repressor bruno plays multiple roles in development and is widely conserved - PubMed (original) (raw)
Translational repressor bruno plays multiple roles in development and is widely conserved
P J Webster et al. Genes Dev. 1997.
Abstract
oskar (osk) mRNA is tightly localized to the posterior pole of the Drosophila oocyte, where the subsequent expression of Osk protein directs abdomen and germ-line formation in the developing embryo. Misplaced expression of Osk protein leads to lethal body patterning defects. The Osk message is translationally repressed before and during the localization process, ensuring that Osk protein is only expressed after the mRNA has reached the posterior. An ovarian protein, Bruno (Bru), has been implicated as a translational repressor of osk mRNA. Here we report the isolation of a cDNA encoding Bru using a novel approach to the expression cloning of an RNA-binding protein, and the identification of previously described mutants in the arrest (aret)-locus as mutants in Bru. The mutant phenotype, along with the binding properties of the protein and its pattern of accumulation within the oocyte, indicate that Bru regulates multiple mRNAs involved in female and male gametogenesis as well as early in embryogenesis. Genetic experiments provide further evidence that Bru functions in the translational repression of osk. Intriguingly, we find that Bru interacts physically with Vasa (Vas), an RNA helicase that is a positive regulator of osk translation. Bru belongs to an evolutionarily conserved family of genes, suggesting that Bru-mediated translational regulation may be widespread. Models for the molecular mechanism of Bru function are discussed.
Figures
Figure 2
bru encodes sex-specific transcripts and protein isoforms. (A) Northern blot hybridized with a bru cDNA probe. (From left to right) Embryonic RNA at 2-hr intervals from 0 to 24 hr after egg laying (first lane, 0–2 hr; second lane, 2–4 hr; etc.); larval RNA from first, second and third instar; pupal RNA in early and late stages of pupal development; and adult RNA from whole males and females. (B) UV cross-linking assays of Drosophila ovary and testis protein extracts with BRE+ and BRE− probes as in Fig. 1A. (C) Western blot of Drosophila ovary, testis and 0- to 2-hr embryo protein extracts probed with α-BruB antibodies; similar results are obtained with α-BruA antibodies (see Materials and Methods). Both isoforms of Bru migrate more slowly than their predicted size.
Figure 1
(A) Identification of a cDNA encoding a BRE-binding activity. Bacterial extracts containing pools of cDNA-encoded ovarian proteins were monitored in UV cross-linking assays for binding to radiolabeled RNA probes containing 16 tandemly repeated copies of either wild-type BREs (+) or BREs in which several nucleotides have been scrambled (−). Only the positive pools containing the binding activity are shown. Complexities of each pool are as follows: (left) 250 clones; (middle) 15 clones; (right) 1 clone. The cDNA-encoded fragment of Bru at the bottom of the gel is progressively more abundant as the complexity of the pool drops. The strong band at the top of the gels (shaded arrowhead) is an E. coli protein that binds nonspecifically to both probes; this protein is not able to compete successfully for the wild-type probe when Bru is abundant (right). (B) The isolated cDNA encodes the ovarian protein Bru. Ovary extracts were cross-linked to a BRE+ RNA probe, followed by the addition of antibodies as indicated. The mobility of the Bru–BRE+ cross-linked product (lane 1, ∼70 kD) is shifted in the presence of antibodies raised against the cDNA-encoded protein (α-BruA; see Materials and Methods; lane 2) but not in the presence of antibodies raised against other Drosophila proteins, including Knirps (lane 3), Bicoid, or Exuperantia (data not shown).
Figure 3
bru encodes two isoforms of an RNA-binding protein conserved in evolution. (A) Deduced peptide sequence of the longest open reading frame in the ovarian bru cDNAs. Predicted RNA-binding domains are shaded. (B) Deduced peptide sequence of the putative amino-terminal domain of testis-specific Bru; the remainder of the protein coincides with residues 29–604 of the ovary-specific form shown in A. (C) Schematic of the protein encoded by the original cDNA and Bru protein isoforms, followed by the amino acid sequence alignment of human RNA-binding protein CUG-BP (GenBank accession no. U63289; Timchenko et al. 1996), Xenopus etr-1 (no. U16800; Knecht et al. 1995), and C. elegans etr-1 (no. U53931; P. Good, pers. comm.) with Bru. With the exception of the fragment encoded by the original cDNA, all sequences represent putative full-length proteins. RNA-binding domains are indicated as boxes, with the percent identity/similarity to the corresponding RNA-binding domains in Bru indicated. There is no striking homology outside of these domains.
Figure 4
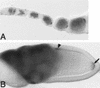
Bru protein colocalizes with osk and grk mRNAs during oogenesis. Distribution of Bru protein in whole-mounted preparations of developing egg chambers. Each egg chamber contains a cluster of nurse cells on the left, and a single oocyte on the right, oriented with the posterior of the oocyte to the right. Bru protein is visualized as a dark stain. (A) Early oogenesis. Bru protein can be seen in all of the germ cells, beginning in stage 2A of the germarium, and is clearly accumulating preferentially in the presumptive oocyte by stage 2B. As with osk mRNA, Bru can be seen as a posterior crescent within the oocyte even in the early stages of oogenesis. Note that Bru protein is also maintained throughout the nurse cells, the site of osk mRNA synthesis, as oogenesis progresses. (B) Stage 10 egg chamber. As with osk mRNA (Ephrussi et al. 1991; Kim-Ha et al. 1991), the posterior localization of Bru in the oocyte becomes more striking in later egg chambers (arrow). Additionally, in stage 10 egg chambers Bru mimics the anterodorsal pattern of grk mRNA localization over the oocyte nucleus (arrowhead; Neuman-Silberberg and Schüpbach 1993).
Figure 5
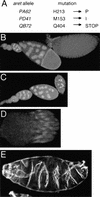
(A) Mutations in aret alleles. Amino acid positions refer to Fig. 3A. (B–D) Whole-mounted tissue stained with DAPI. (B) Wild-type ovariole composed of a string of progressively developing egg chambers, with the oldest egg chamber on the right. (C) Ovariole from an aretPA62/Df(2L)esc-P3-0 female. The progression of oogenesis appears normal until approximately stage 9, when the egg chambers deteriorate. No late-stage eggs are formed or laid. aretPD41/Df(2L)esc-P3-0 females have a similar phenotype. (D) A complete ovary, consisting of 15–20 ovarioles, from an aretQB72/Df(2L)esc-P3-0 female. All ovarioles are arrested in germarial stages. (E) Larval cuticle of an embryo from an aretPA62/aretPD41 mother showing complex defects in the pattern of segmentation (cf. Fig. 6A, wild-type cuticle). The severity of this phenotype is variable; the defects do not resemble the mutant phenotypes resulting from either over- or underexpression of osk.
Figure 6
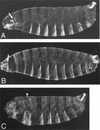
Bru regulates osk activity (A–C) Larval cuticles; anterior is to the left. (A) Wild type; the arrow indicates cephalopharyngeal skeleton. (B) Embryo from a mother carrying transgene P[A7]; anterior expression of transgenic Osk causes defects in the head structures, which can be seen here as a reduction in the cephalopharyngeal skeleton (arrow). (C) Embryo from a mother carrying P[A7] and additionally heterozygous for aretQB72; the P[A7] phenotype is enhanced such that head structures are deleted and replaced with a mirror-image duplication of several abdominal segments. The arrowhead marks the axis of mirror-image duplication. Note that embryos from mothers heterozygous for aretQB72 alone do not show a mutant phenotype (data not shown).
Figure 7
Bru and Vas proteins interact in vitro. Western blot probed with α-BruA antiserum. (Lanes 1,3) Eluate from glutathione–Sepharose 4B beads bound by GST–Vas, challenged with lysate from bacteria expressing a fragment of Bru (BruA; see Materials and Methods), and eluted with SDS sample buffer (lane 1) or factor Xa (lane 3). (Lanes 2,4) Similar beads bound by GST alone, challenged with the BruA lysate, and eluted with SDS sample buffer (lane 2) or factor Xa (lane 4). (Lane 5) Crude BruA-expressing bacterial lysate. Positions of molecular mass markers are indicated at left and correspond to sizes of 138, 86.8, 47.8, 33.3, 28.6 and 20.7 kD.
References
- Bandziulis RJ, Swanson MS, Dreyfuss G. RNA-binding proteins as developmental regulators. Genes & Dev. 1989;3:431–437. - PubMed
- Bass BL, Hurst SR, Singer JD. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr Biol. 1994;4:301–314. - PubMed
- Bouvet P, Omilli F, Arlot-Bonnemains Y, Legagneux V, Roghi C, Bassez T, Osborne HB. The deadenylation conferred by the 3′ untranslated region of a developmentally controlled mRNA in Xenopus embryos is switched to polyadenylation by deletion of a short sequence element. Mol Cell Biol. 1994;14:1893–1900. - PMC - PubMed
- Castrillon DH, Gönczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analyis of spermatogenesis in Drosophila melanogaster: Characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. - PMC - PubMed
- Christerson LB, McKearin DM. orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes & Dev. 1994;8:614–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases