Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway - PubMed (original) (raw)
Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway
G Hu et al. Genes Dev. 1997.
Abstract
DCC (deleted in colorectal cancer) is postulated to function as transmembrane receptor for the axon and cell guidance factor netrin-1. We report here that the DCC cytoplasmic domain binds to proteins encoded by mammalian homologs of the Drosophila seven in absentia (sina) gene, as well as Drosophila Sina. Sina has a critical role in R7 photoreceptor development and shows upward of 85% amino acid identity with its mammalian homologs (termed Siahs), but the function of the Sina/Siah proteins has not been defined. We sought, therefore, to characterize further their interaction with DCC. Immunofluorescence studies suggested the Sina/Siah proteins localized predominantly in the cytoplasm and in association with DCC. DCC was found to be ubiquitinated and the Sina/Siah proteins regulated its expression. Proteasome inhibitors blocked the effects of Sina/Siah on DCC, and the Sina/Siah proteins interacted with ubiquitin-conjugating enzymes (Ubcs). A mutant Siah protein lacking the amino-terminal Ubc-binding sequences complexed with DCC, but did not degrade it. The in vivo interaction between Sina/Siah and DCC was confirmed through studies of transgenic Drosophila lines in which DCC and Sina were ectopically expressed in the eye. Taken together, the data imply that the Sina/Siah proteins regulate DCC and perhaps other proteins via the ubiquitin-proteasome pathway.
Figures
Figure 1
Yeast two-hybrid studies of DCC, Sina, and mammalian Sina homologs (Siahs). (A) Yeast two-hybrid interaction between DCC and Sina/Siah proteins. Yeast strain MaV103 was cotransformed with the indicated GAL4 DNA-binding (DB) and activation domain (AD) fusions. DB fusions were GAL4–DCC4 (DCC4, amino acids 1120–1392) or GAL4DB–p105-RB (RB; amino acids 302–908). AD fusion proteins contained human Siah2Δ1 (amino acids 103–324), mouse Siah2Δ (amino acids 115–325), Drosophila Sina (amino acids 115–314), or human E2F-1 (amino acids 159–437) sequences. Yeast transformants were initially grown on permissive SC–Leu–Trp (master) plates. Reporter assays were performed by replica plating the master plate onto a SC–Leu–Trp–His plate containing 20 m
m
3-AT (−His+3AT 20 m
m
); Hybond N+ membrane on SC–Leu–Trp plate for β-Gal assay (β-Gal); and SC–Leu–Trp plate containing 0.2% 5-fluoro-orotic acid (+URA+5FOA). Positive two-hybrid interactions are indicated by growth on the −HIS + 3AT plate, β-gal activity, and lack of growth on +URA + 5FOA plate. Controls 1–5 are derived from the same yeast strain cotransformed with (1) pPC97–CYH+pPC86; (2) DB–RB+AD–E2F1; (3) DB–Fos+AD–Jun; (4) Gal4+pPC86; and (5) DB-E2F1+AD–DP1. Equivalent expression of the majority of DB and AD fusions was verified by Western blot analysis. (B) Minimal region of Siah-2 needed for interaction with DCC in the yeast two-hybrid assay. Studies to define the Siah-2 sequences were performed as described in A.
Figure 1
Yeast two-hybrid studies of DCC, Sina, and mammalian Sina homologs (Siahs). (A) Yeast two-hybrid interaction between DCC and Sina/Siah proteins. Yeast strain MaV103 was cotransformed with the indicated GAL4 DNA-binding (DB) and activation domain (AD) fusions. DB fusions were GAL4–DCC4 (DCC4, amino acids 1120–1392) or GAL4DB–p105-RB (RB; amino acids 302–908). AD fusion proteins contained human Siah2Δ1 (amino acids 103–324), mouse Siah2Δ (amino acids 115–325), Drosophila Sina (amino acids 115–314), or human E2F-1 (amino acids 159–437) sequences. Yeast transformants were initially grown on permissive SC–Leu–Trp (master) plates. Reporter assays were performed by replica plating the master plate onto a SC–Leu–Trp–His plate containing 20 m
m
3-AT (−His+3AT 20 m
m
); Hybond N+ membrane on SC–Leu–Trp plate for β-Gal assay (β-Gal); and SC–Leu–Trp plate containing 0.2% 5-fluoro-orotic acid (+URA+5FOA). Positive two-hybrid interactions are indicated by growth on the −HIS + 3AT plate, β-gal activity, and lack of growth on +URA + 5FOA plate. Controls 1–5 are derived from the same yeast strain cotransformed with (1) pPC97–CYH+pPC86; (2) DB–RB+AD–E2F1; (3) DB–Fos+AD–Jun; (4) Gal4+pPC86; and (5) DB-E2F1+AD–DP1. Equivalent expression of the majority of DB and AD fusions was verified by Western blot analysis. (B) Minimal region of Siah-2 needed for interaction with DCC in the yeast two-hybrid assay. Studies to define the Siah-2 sequences were performed as described in A.
Figure 2
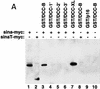
Association of DCC and Sina in vitro. (A) In vitro binding assay for DCC and Sina. Lysates of COS-1 cells transfected with a c-Myc epitope-tagged sina cDNA (Sina–myc, lanes 1,3–7,9), a carboxy-terminal truncated form (SinaT–Myc, lanes 2,8), or empty expression vector (lane 10) were incubated with 10 μg of each affinity–purified GST fusion protein, including GST/DCC-B (amino acids 1155–1447, lanes 3,8,10); GST/DCC-1′ (amino acids 1228–1447, lane 4); GST/DCC-2′ (amino acids 1302–1447, lane 5); GST/DCC-3′ (amino acids 1375–1447, lane 6); GST/DCC–XL (cytoplasmic domain of Xenopus DCC, lane 7); and GST/p16 (human p16 cyclin kinase inhibitor, lane 9). GST fusion proteins were recovered by incubation with glutathione–Sepharose beads, and bound proteins were released and analyzed by Western blot analysis, using the c-Myc monoclonal antibody 9E10.2 and ECL. Lanes 1 and 2 are COS-1 lysates of Sina-Myc and SinaT–Myc, and represent the abundance of the Sina proteins in one-third of the lysate used for each in vitro binding experiment. (B) Mapping of the minimal region of the DCC cytoplasmic domain required for binding to Sina/Siah in yeast and in vitro.
Figure 2
Association of DCC and Sina in vitro. (A) In vitro binding assay for DCC and Sina. Lysates of COS-1 cells transfected with a c-Myc epitope-tagged sina cDNA (Sina–myc, lanes 1,3–7,9), a carboxy-terminal truncated form (SinaT–Myc, lanes 2,8), or empty expression vector (lane 10) were incubated with 10 μg of each affinity–purified GST fusion protein, including GST/DCC-B (amino acids 1155–1447, lanes 3,8,10); GST/DCC-1′ (amino acids 1228–1447, lane 4); GST/DCC-2′ (amino acids 1302–1447, lane 5); GST/DCC-3′ (amino acids 1375–1447, lane 6); GST/DCC–XL (cytoplasmic domain of Xenopus DCC, lane 7); and GST/p16 (human p16 cyclin kinase inhibitor, lane 9). GST fusion proteins were recovered by incubation with glutathione–Sepharose beads, and bound proteins were released and analyzed by Western blot analysis, using the c-Myc monoclonal antibody 9E10.2 and ECL. Lanes 1 and 2 are COS-1 lysates of Sina-Myc and SinaT–Myc, and represent the abundance of the Sina proteins in one-third of the lysate used for each in vitro binding experiment. (B) Mapping of the minimal region of the DCC cytoplasmic domain required for binding to Sina/Siah in yeast and in vitro.
Figure 3
Confocal microscope images of DCC and Sina localization in Drosophila S2 cells and monkey kidney COS-1 cells. Constructs encoding full-length DCC protein (A,E), the DCC cytoplasmic domain (B,F,H) and carboxy-terminal truncated form of the DCC cytoplasmic domain (G) were expressed alone (A,B) or together with Sina (E–H) in Drosophila S2 cells (A–G) or COS-1 cells (H). Full-length Sina (C) or a carboxy-terminal truncated Sina protein (D) were also expressed alone in Drosophila S2 cells. The expression of DCC (red) and Myc-tagged sina (green) was detected by immunofluorescence double staining with a DCC rabbit polyclonal antibody and a c-Myc monoclonal antibody, followed by Texas red-conjugated goat anti-rabbit and FITC-conjugated goat anti-mouse secondary antibodies. The images were captured by confocal microscopy, and pseudo-coloring was performed using the Adobe Photoshop software program. Colocalization of the fluorescence signals was demonstrated by yellow signals when the red and green images were overlaid (e.g., indicated by arrows in F).
Figure 4
Siahs regulate DCC expression in mammalian cells. (A) Schematic representation of DCC and Siah proteins encoded by the cDNA constructs. The truncated forms of Siah-2 are indicated as Siah-2ΔA, SiahΔB, SiahΔC, SiahΔD. (B) Siah-1 and Siah-2 regulate the expression of full-length DCC (DCC-FL), but not that of a truncated form lacking most of the DCC cytoplasmic domain (DCC-T). As indicated, COS-1 and CHO cells were cotransfected with cDNAs for DCC-FL or DCC-T and Siah-1, Siah-2, Siah-2Δ’s, or the control (empty) expression vector. Forty-eight hours after transfection, Western blot analysis was carried out on the cell lysates, using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. The membranes were then stripped and ECL–Western blot analysis was performed with a polyclonal antibody against Na+/K+ ATPase to verify the loading. (C) The expression of a cytoplasmic form of DCC is regulated by Siahs. A cDNA encoding the DCC cytoplasmic domain (DCC-cyto) was cotransfected with Siah-1, Siah-2, or the control CMV expression vector (vector). The Western blot analysis of DCC and Na+/K+ ATPase was performed as above, except that ECL–Western blot analysis of DCC was carried out with DCC cytoplasmic domain monoclonal antibody G97–449. The mobility of molecular weight markers is indicated at the left. (D) Antisense SIAH-2 expression in COS-1 cells results in increased expression of DCC-FL, but has no effect on DCC-T expression. The studies were performed with the indicated vectors as described in B.
Figure 4
Siahs regulate DCC expression in mammalian cells. (A) Schematic representation of DCC and Siah proteins encoded by the cDNA constructs. The truncated forms of Siah-2 are indicated as Siah-2ΔA, SiahΔB, SiahΔC, SiahΔD. (B) Siah-1 and Siah-2 regulate the expression of full-length DCC (DCC-FL), but not that of a truncated form lacking most of the DCC cytoplasmic domain (DCC-T). As indicated, COS-1 and CHO cells were cotransfected with cDNAs for DCC-FL or DCC-T and Siah-1, Siah-2, Siah-2Δ’s, or the control (empty) expression vector. Forty-eight hours after transfection, Western blot analysis was carried out on the cell lysates, using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. The membranes were then stripped and ECL–Western blot analysis was performed with a polyclonal antibody against Na+/K+ ATPase to verify the loading. (C) The expression of a cytoplasmic form of DCC is regulated by Siahs. A cDNA encoding the DCC cytoplasmic domain (DCC-cyto) was cotransfected with Siah-1, Siah-2, or the control CMV expression vector (vector). The Western blot analysis of DCC and Na+/K+ ATPase was performed as above, except that ECL–Western blot analysis of DCC was carried out with DCC cytoplasmic domain monoclonal antibody G97–449. The mobility of molecular weight markers is indicated at the left. (D) Antisense SIAH-2 expression in COS-1 cells results in increased expression of DCC-FL, but has no effect on DCC-T expression. The studies were performed with the indicated vectors as described in B.
Figure 4
Siahs regulate DCC expression in mammalian cells. (A) Schematic representation of DCC and Siah proteins encoded by the cDNA constructs. The truncated forms of Siah-2 are indicated as Siah-2ΔA, SiahΔB, SiahΔC, SiahΔD. (B) Siah-1 and Siah-2 regulate the expression of full-length DCC (DCC-FL), but not that of a truncated form lacking most of the DCC cytoplasmic domain (DCC-T). As indicated, COS-1 and CHO cells were cotransfected with cDNAs for DCC-FL or DCC-T and Siah-1, Siah-2, Siah-2Δ’s, or the control (empty) expression vector. Forty-eight hours after transfection, Western blot analysis was carried out on the cell lysates, using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. The membranes were then stripped and ECL–Western blot analysis was performed with a polyclonal antibody against Na+/K+ ATPase to verify the loading. (C) The expression of a cytoplasmic form of DCC is regulated by Siahs. A cDNA encoding the DCC cytoplasmic domain (DCC-cyto) was cotransfected with Siah-1, Siah-2, or the control CMV expression vector (vector). The Western blot analysis of DCC and Na+/K+ ATPase was performed as above, except that ECL–Western blot analysis of DCC was carried out with DCC cytoplasmic domain monoclonal antibody G97–449. The mobility of molecular weight markers is indicated at the left. (D) Antisense SIAH-2 expression in COS-1 cells results in increased expression of DCC-FL, but has no effect on DCC-T expression. The studies were performed with the indicated vectors as described in B.
Figure 4
Siahs regulate DCC expression in mammalian cells. (A) Schematic representation of DCC and Siah proteins encoded by the cDNA constructs. The truncated forms of Siah-2 are indicated as Siah-2ΔA, SiahΔB, SiahΔC, SiahΔD. (B) Siah-1 and Siah-2 regulate the expression of full-length DCC (DCC-FL), but not that of a truncated form lacking most of the DCC cytoplasmic domain (DCC-T). As indicated, COS-1 and CHO cells were cotransfected with cDNAs for DCC-FL or DCC-T and Siah-1, Siah-2, Siah-2Δ’s, or the control (empty) expression vector. Forty-eight hours after transfection, Western blot analysis was carried out on the cell lysates, using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. The membranes were then stripped and ECL–Western blot analysis was performed with a polyclonal antibody against Na+/K+ ATPase to verify the loading. (C) The expression of a cytoplasmic form of DCC is regulated by Siahs. A cDNA encoding the DCC cytoplasmic domain (DCC-cyto) was cotransfected with Siah-1, Siah-2, or the control CMV expression vector (vector). The Western blot analysis of DCC and Na+/K+ ATPase was performed as above, except that ECL–Western blot analysis of DCC was carried out with DCC cytoplasmic domain monoclonal antibody G97–449. The mobility of molecular weight markers is indicated at the left. (D) Antisense SIAH-2 expression in COS-1 cells results in increased expression of DCC-FL, but has no effect on DCC-T expression. The studies were performed with the indicated vectors as described in B.
Figure 5
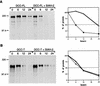
Siah-2 decreases DCC half-life via its cytoplasmic domain. A pulse–chase analysis was undertaken to determine the half-life of DCC proteins in the presence or absence of Siah-2. COS-1 cells were cotransfected with expression constructs encoding full-length DCC (DCC-FL, A) or a truncated form of DCC lacking the majority of the cytoplasmic domain (DCC-T, B) and human Siah-2 (Siah-2) or the control expression vector. Forty-eight hours after transfection, the cells were pulse-labeled for 1 hr with a [35S]methione/cysteine mix, chased with cold methione/cysteine for the indicated times, and then lysed. DCC proteins were immunoprecipitated with polyclonal antibody 645 and analyzed by SDS-PAGE and fluorography (left). The mobility of molecular weight markers is indicated. The autoradiographic signals of the ∼190 kD DCC protein were quantitated by densitometry and the data were plotted as a function of time (right) (○) DCC-FL; (▪) DCC-FL and Siah-2 in A. (▵) DCC-T; (•) DCC-T and Siah-2 in B.
Figure 6
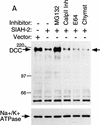
Effects of Siah-2 on DCC expression are blocked by the proteasome inhibitor MG132. (A) Forty-eight hours after co-transfection of COS-1 cells with expression vectors encoding full-length DCC and Siah-2 or a control vector, cells were incubated with dimethyl sulfoxide (DMSO; − inhibitor lane) or various protease inhibitors for 6 hr at 37°C. The final concentrations of the inhibitors in the media were: MG132, 20 μ
m
; Calpain inhibitor II (Calp II Inh), 20 μ
m
; E64, 50 μ
m
; chymostatin (Chymst), 50 μ
m
. After incubation, lysates were prepared and the levels of DCC expression were detected by Western blot analysis using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. Membranes were then stripped and Western blotted with an antibody against Na+/K+ ATPase to verify loading. Mobility of molecular weight markers is indicated at the left. (B) Dose response of MG132 inhibition of Siah-2-mediated DCC degradation. The studies were carried out as described in A.
Figure 6
Effects of Siah-2 on DCC expression are blocked by the proteasome inhibitor MG132. (A) Forty-eight hours after co-transfection of COS-1 cells with expression vectors encoding full-length DCC and Siah-2 or a control vector, cells were incubated with dimethyl sulfoxide (DMSO; − inhibitor lane) or various protease inhibitors for 6 hr at 37°C. The final concentrations of the inhibitors in the media were: MG132, 20 μ
m
; Calpain inhibitor II (Calp II Inh), 20 μ
m
; E64, 50 μ
m
; chymostatin (Chymst), 50 μ
m
. After incubation, lysates were prepared and the levels of DCC expression were detected by Western blot analysis using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. Membranes were then stripped and Western blotted with an antibody against Na+/K+ ATPase to verify loading. Mobility of molecular weight markers is indicated at the left. (B) Dose response of MG132 inhibition of Siah-2-mediated DCC degradation. The studies were carried out as described in A.
Figure 7
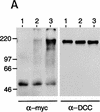
DCC, Sina/Siah, the ubiquitin pathway, and stable interactions between DCC and a mutant form of Siah-1. (A) DCC is ubiquitinated. COS-1 cells were cotransfected with vectors encoding full-length DCC (lanes 1–3), a c-Myc epitope-tagged yeast ubiquitin (lanes 2,3), Siah-2 (lane 3), and control vector (lane 1). Forty-eight hours after transfection, the cells were treated for 6 hr at 37°C with 20 μ
m
MG132 dissolved in DMSO (lane 3) or the same concentration of DMSO (lane 1 and 2). DCC proteins were collected by immunoprecipitation with DCC polyclonal antibodies 645 and 723. The immunoprecipitates were electrophoresed and Western blot analysis was performed to identify DCC proteins to which the c-Myc tagged ubiquitin polypeptide had been covalently attached, using c-Myc monoclonal antibody 9E10.2 and ECL reagents (left panel). After stripping the blot, the abundance of DCC protein in each lane was determined by Western blot analysis with DCC extracellular domain monoclonal antibody G92-13 and ECL reagents (right panel). The mobility of molecular weight markers is indicated at the left. (B) Sina and human Siah homologs interact with ubiquitin-conjugating (Ubc) proteins in the yeast two-hybrid system. DB fusion constructs and AD fusion constructs were cotransformed into yeast strain MaV103. (DB fusions) Full-length human Siah1 and Siah2 (hSiah1 and hSiah2); full-length Drosophila Sina (sina); amino-terminal truncated Sina (sinaT); human DCC cytoplasmic domain (DCC4, amino acids 1120–1392); human retinoblastoma protein (Rb); truncated form of human neogenin cytoplasmic domain (Neo-T); spliced form of human neogenin cytoplasmic domain (NeoS); and the truncated and spliced form of human neogenin cytoplasmic domain (NeoS-T). (AD fusions) Human homolog of yeast UBC9 (hUBC) and Drosophila homolog of UBC9 (Dubc). Yeast transformations, growth, reporter gene studies, and interaction controls 1–5 were as described in Materials and Methods and the legend to Fig. 1. (C) Siah-1 protein lacking amino-terminal Ubc-binding sequences forms a complex with DCC in cells. CHO cells were transfected with vectors encoding the indicated proteins. The pcDNA3 construct for the FLAG epitope-tagged, truncated Siah-1 (i.e., FLAG–Siah1Δ) has the FLAG peptide tag MDYKDDDDK fused to amino acids 77–282 of Siah-1. Lysates were prepared 48 hr after transfection, and a portion of the lysate was used for immunoprecipitation with DCC polyclonal antibody 645, directed against the DCC extracellular domain. The lysates (lanes 1–4) and immunoprecipitates (lanes 5–8) were electrophoresed and ECL–Western blotting was carried out with a monoclonal antibody against the extracellular domain of DCC or against the FLAG epitope. Siah-1 coprecipitated with full-length DCC (DCC-FL) (lane 5) but not with a truncated form of DCC (DCC-T), lacking the DCC cytoplasmic domain (lane 8). The mobilities of molecular weight standards and the DCC and FLAG–Siah1Δ proteins are indicated.
Figure 7
DCC, Sina/Siah, the ubiquitin pathway, and stable interactions between DCC and a mutant form of Siah-1. (A) DCC is ubiquitinated. COS-1 cells were cotransfected with vectors encoding full-length DCC (lanes 1–3), a c-Myc epitope-tagged yeast ubiquitin (lanes 2,3), Siah-2 (lane 3), and control vector (lane 1). Forty-eight hours after transfection, the cells were treated for 6 hr at 37°C with 20 μ
m
MG132 dissolved in DMSO (lane 3) or the same concentration of DMSO (lane 1 and 2). DCC proteins were collected by immunoprecipitation with DCC polyclonal antibodies 645 and 723. The immunoprecipitates were electrophoresed and Western blot analysis was performed to identify DCC proteins to which the c-Myc tagged ubiquitin polypeptide had been covalently attached, using c-Myc monoclonal antibody 9E10.2 and ECL reagents (left panel). After stripping the blot, the abundance of DCC protein in each lane was determined by Western blot analysis with DCC extracellular domain monoclonal antibody G92-13 and ECL reagents (right panel). The mobility of molecular weight markers is indicated at the left. (B) Sina and human Siah homologs interact with ubiquitin-conjugating (Ubc) proteins in the yeast two-hybrid system. DB fusion constructs and AD fusion constructs were cotransformed into yeast strain MaV103. (DB fusions) Full-length human Siah1 and Siah2 (hSiah1 and hSiah2); full-length Drosophila Sina (sina); amino-terminal truncated Sina (sinaT); human DCC cytoplasmic domain (DCC4, amino acids 1120–1392); human retinoblastoma protein (Rb); truncated form of human neogenin cytoplasmic domain (Neo-T); spliced form of human neogenin cytoplasmic domain (NeoS); and the truncated and spliced form of human neogenin cytoplasmic domain (NeoS-T). (AD fusions) Human homolog of yeast UBC9 (hUBC) and Drosophila homolog of UBC9 (Dubc). Yeast transformations, growth, reporter gene studies, and interaction controls 1–5 were as described in Materials and Methods and the legend to Fig. 1. (C) Siah-1 protein lacking amino-terminal Ubc-binding sequences forms a complex with DCC in cells. CHO cells were transfected with vectors encoding the indicated proteins. The pcDNA3 construct for the FLAG epitope-tagged, truncated Siah-1 (i.e., FLAG–Siah1Δ) has the FLAG peptide tag MDYKDDDDK fused to amino acids 77–282 of Siah-1. Lysates were prepared 48 hr after transfection, and a portion of the lysate was used for immunoprecipitation with DCC polyclonal antibody 645, directed against the DCC extracellular domain. The lysates (lanes 1–4) and immunoprecipitates (lanes 5–8) were electrophoresed and ECL–Western blotting was carried out with a monoclonal antibody against the extracellular domain of DCC or against the FLAG epitope. Siah-1 coprecipitated with full-length DCC (DCC-FL) (lane 5) but not with a truncated form of DCC (DCC-T), lacking the DCC cytoplasmic domain (lane 8). The mobilities of molecular weight standards and the DCC and FLAG–Siah1Δ proteins are indicated.
Figure 7
DCC, Sina/Siah, the ubiquitin pathway, and stable interactions between DCC and a mutant form of Siah-1. (A) DCC is ubiquitinated. COS-1 cells were cotransfected with vectors encoding full-length DCC (lanes 1–3), a c-Myc epitope-tagged yeast ubiquitin (lanes 2,3), Siah-2 (lane 3), and control vector (lane 1). Forty-eight hours after transfection, the cells were treated for 6 hr at 37°C with 20 μ
m
MG132 dissolved in DMSO (lane 3) or the same concentration of DMSO (lane 1 and 2). DCC proteins were collected by immunoprecipitation with DCC polyclonal antibodies 645 and 723. The immunoprecipitates were electrophoresed and Western blot analysis was performed to identify DCC proteins to which the c-Myc tagged ubiquitin polypeptide had been covalently attached, using c-Myc monoclonal antibody 9E10.2 and ECL reagents (left panel). After stripping the blot, the abundance of DCC protein in each lane was determined by Western blot analysis with DCC extracellular domain monoclonal antibody G92-13 and ECL reagents (right panel). The mobility of molecular weight markers is indicated at the left. (B) Sina and human Siah homologs interact with ubiquitin-conjugating (Ubc) proteins in the yeast two-hybrid system. DB fusion constructs and AD fusion constructs were cotransformed into yeast strain MaV103. (DB fusions) Full-length human Siah1 and Siah2 (hSiah1 and hSiah2); full-length Drosophila Sina (sina); amino-terminal truncated Sina (sinaT); human DCC cytoplasmic domain (DCC4, amino acids 1120–1392); human retinoblastoma protein (Rb); truncated form of human neogenin cytoplasmic domain (Neo-T); spliced form of human neogenin cytoplasmic domain (NeoS); and the truncated and spliced form of human neogenin cytoplasmic domain (NeoS-T). (AD fusions) Human homolog of yeast UBC9 (hUBC) and Drosophila homolog of UBC9 (Dubc). Yeast transformations, growth, reporter gene studies, and interaction controls 1–5 were as described in Materials and Methods and the legend to Fig. 1. (C) Siah-1 protein lacking amino-terminal Ubc-binding sequences forms a complex with DCC in cells. CHO cells were transfected with vectors encoding the indicated proteins. The pcDNA3 construct for the FLAG epitope-tagged, truncated Siah-1 (i.e., FLAG–Siah1Δ) has the FLAG peptide tag MDYKDDDDK fused to amino acids 77–282 of Siah-1. Lysates were prepared 48 hr after transfection, and a portion of the lysate was used for immunoprecipitation with DCC polyclonal antibody 645, directed against the DCC extracellular domain. The lysates (lanes 1–4) and immunoprecipitates (lanes 5–8) were electrophoresed and ECL–Western blotting was carried out with a monoclonal antibody against the extracellular domain of DCC or against the FLAG epitope. Siah-1 coprecipitated with full-length DCC (DCC-FL) (lane 5) but not with a truncated form of DCC (DCC-T), lacking the DCC cytoplasmic domain (lane 8). The mobilities of molecular weight standards and the DCC and FLAG–Siah1Δ proteins are indicated.
Figure 8
Ectopic expression of human DCC in the Drosophila eye causes rough eye phenotypes. Full-length human DCC cDNA coding sequence were expressed in transgenic flies under the transcriptional control of Drosophila sev promoter and enhancer elements. Shown are low (left panels) and high (right panels) power scanning electron micrographs of adult Drosophila eyes: Wild type (A,B); 2× sev–DCC (C,D); 4× sev–DCC (E,F).
Figure 9
Ommatidial defects are seen in apical retinal sections from sev–DCC flies. Low power (left panels) and high power (right panels) images of apical retinal sections from flies with various genotypes are shown: Wild type (A,B); 2× sev–DCC (C,D); 4× sev–DCC (E,F); and 2× sev–DCC, 1× sev–sina (G,H). Ommatidia with R7 photoreceptor cells missing are indicated by white arrows in D; the arrowhead indicates an ommatidia missing an outer photoreceptor cell.
References
- Brunner D, Oellers N, Szabad J, Biggs III WH, Zipursky SL, Hafen E. A gain of function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. - PubMed
- Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. - PubMed
- Cavenee WK, Dryja TP, Phillips RA, Benedict WF, Godbout R, Gallie GL, Murphree AL, Strong LC, White RL. Expression of recessive alleles by chromosomal mechansims in retinoblastoma. Nature. 1983;305:779–784. - PubMed
- Chan SS-Y, Zheng H, Su M-W, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases