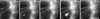Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling - PubMed (original) (raw)
Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling
C M Waterman-Storer et al. J Cell Biol. 1997.
Abstract
We have discovered several novel features exhibited by microtubules (MTs) in migrating newt lung epithelial cells by time-lapse imaging of fluorescently labeled, microinjected tubulin. These cells exhibit leading edge ruffling and retrograde flow in the lamella and lamellipodia. The plus ends of lamella MTs persist in growth perpendicular to the leading edge until they reach the base of the lamellipodium, where they oscillate between short phases of growth and shortening. Occasionally "pioneering" MTs grow into the lamellipodium, where microtubule bending and reorientation parallel to the leading edge is associated with retrograde flow. MTs parallel to the leading edge exhibit significantly different dynamics from MTs perpendicular to the cell edge. Both parallel MTs and photoactivated fluorescent marks on perpendicular MTs move rearward at the 0.4 mircon/min rate of retrograde flow in the lamella. MT rearward transport persists when MT dynamic instability is inhibited by 100-nM nocodazole but is blocked by inhibition of actomyosin by cytochalasin D or 2,3-butanedione-2-monoxime. Rearward flow appears to cause MT buckling and breaking in the lamella. 80% of free minus ends produced by breakage are stable; the others shorten and pause, leading to MT treadmilling. Free minus ends of unknown origin also depolymerize into the field of view at the lamella. Analysis of MT dynamics at the centrosome shows that these minus ends do not arise by centrosomal ejection and that approximately 80% of the MTs in the lamella are not centrosome bound. We propose that actomyosin-based retrograde flow of MTs causes MT breakage, forming quasi-stable noncentrosomal MTs whose turnover is regulated primarily at their minus ends.
Figures
Figure 1
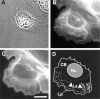
Cytoskeletal architecture of a migrating newt lung epithelial cell. Cells were fixed and processed for immunofluorescent localization of tubulin to visualize MTs (B) and stained with Texas red-phalloidin to localize F-actin (C). MTs are relatively straight and extend radially from the centrosome, while in the cell body they are very sinuous. In the lamella, MTs are generally oriented either parallel or perpendicular to the cell edge. F-actin is concentrated in the lamella and lamellipodia. In D the outline of the phase image of the cell (A) was traced, and the regions of the cell referred to in the text are defined as follows: Nu, nucleus; La, lamella (between gray dotted and dashed lines); Lp, lamellipodia (between gray dashed line and cell edge); CB, cell body (behind thick dotted line); thick gray dotted line, base of the lamella; thick gray dashed line, base of lamellipodia; arrows show the direction of retrograde flow. Several examples of perpendicular MTs (thin white lines) and parallel MTs (thin white dashed lines) are traced from the image in B as well. Bar, 15 μm.
Figure 2
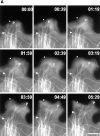
Dynamics of MTs in the lamella and lamellipodia during protrusion of the leading edge. (A) A series of fluorescence micrographs of the leading edge of a cell that had been injected with X-rhodamine–labeled tubulin, elapsed time in min/sec in the upper right of each panel. The boundary of the cell can be seen in negative image because labeled tubulin subunits are diffusely fluorescent within the cell but not outside of the cell. Before protrusion of the cell edge (times 00:00–00:39) most MTs extend only as far as the one marked with an arrowhead, however some MTs, such as the one marked P, extend closer to the leading edge. As the leading edge advances (times 00:39–04:49), the plus end of the P MT approximately maintains its distance from the leading edge, while the MT at the arrowhead does not extend into the new protrusion until advancement of the leading edge has ceased (times 4:49–5:29). (B) Dynamic life history plots of the plus ends of the MTs marked in A in relation to the position of the cell edge. Distance from the origin (a point at the bottom edge of the micrograph at time 00:00) was plotted against time for images captured at 7-s intervals. The position of the edge was determined from fluorescence intensity linescans perpendicular to and across the leading edge. The “pioneer” MT steadily grows at about 2 μm/min during advancement of the leading edge (∼0–5 min). The proximal perpendicular MT undergoes very little net growth until advancement of the leading edge ceases (∼5 min), whereupon the MT rapidly grows at ∼8 μm/min. Bar, 10 μm.
Figure 2
Dynamics of MTs in the lamella and lamellipodia during protrusion of the leading edge. (A) A series of fluorescence micrographs of the leading edge of a cell that had been injected with X-rhodamine–labeled tubulin, elapsed time in min/sec in the upper right of each panel. The boundary of the cell can be seen in negative image because labeled tubulin subunits are diffusely fluorescent within the cell but not outside of the cell. Before protrusion of the cell edge (times 00:00–00:39) most MTs extend only as far as the one marked with an arrowhead, however some MTs, such as the one marked P, extend closer to the leading edge. As the leading edge advances (times 00:39–04:49), the plus end of the P MT approximately maintains its distance from the leading edge, while the MT at the arrowhead does not extend into the new protrusion until advancement of the leading edge has ceased (times 4:49–5:29). (B) Dynamic life history plots of the plus ends of the MTs marked in A in relation to the position of the cell edge. Distance from the origin (a point at the bottom edge of the micrograph at time 00:00) was plotted against time for images captured at 7-s intervals. The position of the edge was determined from fluorescence intensity linescans perpendicular to and across the leading edge. The “pioneer” MT steadily grows at about 2 μm/min during advancement of the leading edge (∼0–5 min). The proximal perpendicular MT undergoes very little net growth until advancement of the leading edge ceases (∼5 min), whereupon the MT rapidly grows at ∼8 μm/min. Bar, 10 μm.
Figure 3
Bending, reorientation, and retrograde flow of a MT in the lamellipodia. (A) A series of micrographs in which the fluorescence image of X-rhodamine–labeled MTs (pseudocolored red) has been digitally superimposed onto the DIC image (in grayscale) of the lamella. Pairs of fluorescence and DIC images were captured within 1.5 s of each other at 9-s intervals; elapsed time in min/sec is in the lower right of each panel. The base of the lamellipodia can be seen as a slightly diffuse staining of X-rhodamine–labeled subunits ∼5 μm from the leading edge. Because the cell is slightly thicker in this region (not shown), the increased volume produces a higher amount of fluorescent label. (B) Dynamic life history plot of the distance of the MT end at the arrowhead in A from the origin (the position of the plus end at time 00:00) versus time. (C) Plot of the distance of a point on the MT (square in A) from the leading edge (directly in front of the point) versus time. The y axis is inverted for clarity. Initially, the MT plus end (arrowhead) perpendicular to the leading edge exhibits little net growth (section 1 of graph B). The MT then grows from within the lamella into the lamellipodia and touches the plasma membrane (time 00:00–01:56 in A; section 2 of graph B) and then undergoes dynamic instability as it “probes” the leading edge (times 01:56–03:52 in A; section 3 of graph B) and then bends within the lamellipodia (times 03:52–05:32 in A; section 4 of graph B), reestablishing its axis of growth parallel to the leading edge. The plus end then undergoes rapid net growth (times 05:32–07:28 in A; section 5 of graph B). The parallel portion of the MT (black square) then moves rearward away from the leading edge (times 6:17–8:58 in A; graph C). Bar, 10 μm.
Figure 3
Bending, reorientation, and retrograde flow of a MT in the lamellipodia. (A) A series of micrographs in which the fluorescence image of X-rhodamine–labeled MTs (pseudocolored red) has been digitally superimposed onto the DIC image (in grayscale) of the lamella. Pairs of fluorescence and DIC images were captured within 1.5 s of each other at 9-s intervals; elapsed time in min/sec is in the lower right of each panel. The base of the lamellipodia can be seen as a slightly diffuse staining of X-rhodamine–labeled subunits ∼5 μm from the leading edge. Because the cell is slightly thicker in this region (not shown), the increased volume produces a higher amount of fluorescent label. (B) Dynamic life history plot of the distance of the MT end at the arrowhead in A from the origin (the position of the plus end at time 00:00) versus time. (C) Plot of the distance of a point on the MT (square in A) from the leading edge (directly in front of the point) versus time. The y axis is inverted for clarity. Initially, the MT plus end (arrowhead) perpendicular to the leading edge exhibits little net growth (section 1 of graph B). The MT then grows from within the lamella into the lamellipodia and touches the plasma membrane (time 00:00–01:56 in A; section 2 of graph B) and then undergoes dynamic instability as it “probes” the leading edge (times 01:56–03:52 in A; section 3 of graph B) and then bends within the lamellipodia (times 03:52–05:32 in A; section 4 of graph B), reestablishing its axis of growth parallel to the leading edge. The plus end then undergoes rapid net growth (times 05:32–07:28 in A; section 5 of graph B). The parallel portion of the MT (black square) then moves rearward away from the leading edge (times 6:17–8:58 in A; graph C). Bar, 10 μm.
Figure 4
Rearward movement of surface-coupled beads and parallel MTs in the lamella. (A) Digitally superimposed fluorescence images (acquired within 1.5 s of each other) of a cell injected with X-rhodamine tubulin (red) that was mounted in media containing 1 μm aminated Cascade blue latex beads (light blue). Elapsed time in min/sec in the upper right of each panel. The bead denoted by the blue triangle is attached to the cell surface and moves rearward while overlying a parallel MT within the cell (green square). During the time period, the leading edge of the cell advanced. (B) Graph of the distance between the bead (light blue triangle) or parallel MTs (green square and yellow circle) and the leading edge of the cell in A versus time (images taken at 3-min intervals). The y axis is inverted for clarity. All three markers move away from the leading edge with identical velocities. Bar, 10 μm.
Figure 4
Rearward movement of surface-coupled beads and parallel MTs in the lamella. (A) Digitally superimposed fluorescence images (acquired within 1.5 s of each other) of a cell injected with X-rhodamine tubulin (red) that was mounted in media containing 1 μm aminated Cascade blue latex beads (light blue). Elapsed time in min/sec in the upper right of each panel. The bead denoted by the blue triangle is attached to the cell surface and moves rearward while overlying a parallel MT within the cell (green square). During the time period, the leading edge of the cell advanced. (B) Graph of the distance between the bead (light blue triangle) or parallel MTs (green square and yellow circle) and the leading edge of the cell in A versus time (images taken at 3-min intervals). The y axis is inverted for clarity. All three markers move away from the leading edge with identical velocities. Bar, 10 μm.
Figure 5
Rearward movement of the lattice of perpendicular MTs in the lamella. (A) Digitally overlaid and pseudocolored micrographs of a cell that was injected with a mixture of X-rhodamine (red)- and caged fluorescein (yellow–green)-labeled tubulins (captured at 3-min intervals within 1.5 s of each other). The cell was exposed to a 2.5-μm-wide bar of UV light to activate the fluorescein label just before the first image. Elapsed time (in min/ sec) is in the upper left of each panel. (B) Plots of relative fluorescence intensity (after background subtraction) versus position along the white line in A. Fluorescence loss due to photobleaching of C2CF during the total exposure time is <5% under similar conditions (not shown). The green lines represent intensity of uncaged fluorescein, and the red lines represent intensity of X-rhodamine. Time at which the scan was taken is denoted by the thickness of the plotted line. The position of the fluorescein-labeled subunits in the primarily perpendicular MTs moves rearward through the lamella over time (A, white arrowheads in B over the green scanlines mark the peak fluorescein intensity), while the level of the X-rhodamine–labeled MT polymer remains relatively constant across the lamella over time (red scan lines in B). Loss in intensity of the fluorescein signal over time is due to depolymerization of MTs through the marked region and photobleaching. Note that the fluorescein-labeled subunits move rearward as a relatively coherent bar (A), and the width of the bar increases very little over time (B). Bar, 10 μm.
Figure 5
Rearward movement of the lattice of perpendicular MTs in the lamella. (A) Digitally overlaid and pseudocolored micrographs of a cell that was injected with a mixture of X-rhodamine (red)- and caged fluorescein (yellow–green)-labeled tubulins (captured at 3-min intervals within 1.5 s of each other). The cell was exposed to a 2.5-μm-wide bar of UV light to activate the fluorescein label just before the first image. Elapsed time (in min/ sec) is in the upper left of each panel. (B) Plots of relative fluorescence intensity (after background subtraction) versus position along the white line in A. Fluorescence loss due to photobleaching of C2CF during the total exposure time is <5% under similar conditions (not shown). The green lines represent intensity of uncaged fluorescein, and the red lines represent intensity of X-rhodamine. Time at which the scan was taken is denoted by the thickness of the plotted line. The position of the fluorescein-labeled subunits in the primarily perpendicular MTs moves rearward through the lamella over time (A, white arrowheads in B over the green scanlines mark the peak fluorescein intensity), while the level of the X-rhodamine–labeled MT polymer remains relatively constant across the lamella over time (red scan lines in B). Loss in intensity of the fluorescein signal over time is due to depolymerization of MTs through the marked region and photobleaching. Note that the fluorescein-labeled subunits move rearward as a relatively coherent bar (A), and the width of the bar increases very little over time (B). Bar, 10 μm.
Figure 6
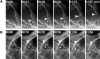
Rearward flow of assembly-inhibited MT plus ends in the lamella. (A) Selected fluorescence micrographs from a series of a cell that was injected with X-rhodamine–labeled tubulin and then mounted in media containing 100 nM nocodazole to inhibit MT plus end assembly dynamics. Elapsed time after addition of nocodazole shown in the upper right of each panel. The positions of three MT plus ends are highlighted. (B) Dynamic life history plots of the distance of the plus ends of the three MTs marked in A from the leading edge of the cell versus time (images acquired at 7-s intervals). The y axis is inverted for clarity. The MT plus ends do not exhibit typical plus end dynamic instability but instead move slowly away from the cell edge at ∼0.4 μm/min (B). While moving rearward, the MTs maintain characteristic bending patterns (A), indicating that the movement of the plus end is not due to depolymerization. Bar, 10 μm.
Figure 6
Rearward flow of assembly-inhibited MT plus ends in the lamella. (A) Selected fluorescence micrographs from a series of a cell that was injected with X-rhodamine–labeled tubulin and then mounted in media containing 100 nM nocodazole to inhibit MT plus end assembly dynamics. Elapsed time after addition of nocodazole shown in the upper right of each panel. The positions of three MT plus ends are highlighted. (B) Dynamic life history plots of the distance of the plus ends of the three MTs marked in A from the leading edge of the cell versus time (images acquired at 7-s intervals). The y axis is inverted for clarity. The MT plus ends do not exhibit typical plus end dynamic instability but instead move slowly away from the cell edge at ∼0.4 μm/min (B). While moving rearward, the MTs maintain characteristic bending patterns (A), indicating that the movement of the plus end is not due to depolymerization. Bar, 10 μm.
Figure 7
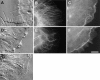
Affects of cytochalasin D and BDM on the architecture and cytoskeleton of the lamella and lamellipodia. VE-DIC images of the lamella of living cells (A and D) ∼5 min after the perfusion of 2.5 μM cytochalasin D (A) or 20 mM BDM (D) and an untreated cell (G), for comparison. In A, the leading edge formed protrusions as the plasma membrane retracted around growing MTs. In D, the lamellipodia continued to ruffle and exhibit retrograde flow, but this motility ended abruptly at the margin at the base of the lamellipodia. Within the lamella, retrograde flow was inhibited. Fluorescence images of microtubules (B and E, with anti-tubulin antibodies) and F-actin (C and F, with Texas red-phalloidin) in fixed cells treated for 20 min with 2.5 μM cytochalasin D (B and C) or for 5 min with 20 mM BDM (C and F). Treatment with cytochalasin D caused the lamellipodia to fill up with microtubules and for F-actin to concentrate into large puncta. Preservation of the membrane extensions shown by VE-DIC in A by fixation was not possible. Treatment with BDM left the microtubule array slightly bundled but relatively undisturbed and resulted in a large concentration of F-actin at the actin marginal band. Bar, 10 μm.
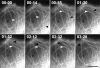
Figure 8
MT breakage induced by local MT buckling in the lamella. Selected images from series of fluorescence micrographs of cells injected with X-rhodamine–labeled tubulin. Time in min/ sec is shown in the upper left of each panel. (A) A MT with local buckling broke at 11 s. The newly formed minus end (large arrowhead) shortened immediately after breakage, while the new plus end (small arrowhead) formed by the break shortened slightly before beginning to undergo dynamic instability (times 00:19–1:57). (B) A MT with local buckles broke (time = 00:10), forming a new minus end (large arrowhead) that remained stable and a new plus end that shortened before undergoing dynamic instability (times 00:20–01:59). Bar, 10 μm.
Figure 9
MT minus ends depolymerize into the field of view in the lamella. Fluorescence images from a series acquired at 7-s intervals of a cell injected with X-rhodamine–labeled tubulin. Elapsed time in min/sec is in the upper left of each panel. The leading edge of the cell is visible in negative image near the top of each panel. The positions of two different MT minus ends are highlighted with white and black arrowheads, respectively, in each panel. The minus end at the white arrow was present at the start of the sequence. The MT rapidly depolymerizes from the minus end (times 00:00–00:55) which then stabilizes and remains so (times 00:55–02:32) until the MT is consumed by plus end depolymerization (time 03:28). The minus end at the white arrow enters the field of view by rapid depolymerization (times 00:14– 00:55), which it continues until the MT is nearly consumed (times 00:55–03:28). Note that the plus end of this MT grows (times 02:12–03:28); thus a MT piece appears to move in the lamella as the minus end continues to depolymerize during the same time period. Bar, 10 μm.
Figure 10
Treadmilling of a MT in the lamella. (A) Fluorescence micrographs (taken at 9-s intervals) of a region in the lamella of a cell that had been injected with a relatively low level of X-rhodamine–labeled tubulin. The featured MT was parallel to and ∼8 μm from the leading edge of the cell, which was at the right. The low level of labeled tubulin results in MTs that are unevenly fluorescent along their lengths. The position of the plus (top) and minus (bottom) ends of the MT are marked with white arrowheads, while the position of a dark (unlabeled) region of the MT lattice is marked with a black arrowhead. The polarity of the MT ends was identified when the MT broke (shown in the previous micrographs). The treadmilling MT was also undergoing retrograde flow toward the cell body (to the left). (B) Graph of the distance of the minus end, the plus end, and the dark mark in the MT lattice versus time. Distance was measured relative to the minus end of the MT. The arrow spans the portion of the graph represented in the micrographs in A. The plus end of the MT undergoes growth and shortening assembly dynamics typical of parallel MTs; long excursions of uninterrupted growth interspersed with short periods of pause or shortening. The minus end rapidly shortens, pauses for a relatively long period of time, and then resumes rapid shortening. The dark region of the lattice stays relatively stationary with respect to either end, with slight translocations of the lattice occurring at around 30–40 and 70 s (in graph B). Bar, 2 μm.
Figure 10
Treadmilling of a MT in the lamella. (A) Fluorescence micrographs (taken at 9-s intervals) of a region in the lamella of a cell that had been injected with a relatively low level of X-rhodamine–labeled tubulin. The featured MT was parallel to and ∼8 μm from the leading edge of the cell, which was at the right. The low level of labeled tubulin results in MTs that are unevenly fluorescent along their lengths. The position of the plus (top) and minus (bottom) ends of the MT are marked with white arrowheads, while the position of a dark (unlabeled) region of the MT lattice is marked with a black arrowhead. The polarity of the MT ends was identified when the MT broke (shown in the previous micrographs). The treadmilling MT was also undergoing retrograde flow toward the cell body (to the left). (B) Graph of the distance of the minus end, the plus end, and the dark mark in the MT lattice versus time. Distance was measured relative to the minus end of the MT. The arrow spans the portion of the graph represented in the micrographs in A. The plus end of the MT undergoes growth and shortening assembly dynamics typical of parallel MTs; long excursions of uninterrupted growth interspersed with short periods of pause or shortening. The minus end rapidly shortens, pauses for a relatively long period of time, and then resumes rapid shortening. The dark region of the lattice stays relatively stationary with respect to either end, with slight translocations of the lattice occurring at around 30–40 and 70 s (in graph B). Bar, 2 μm.
Figure 11
Dynamics of MTs at the centrosome. Fluorescence images from a series (taken at 7-s intervals) of a cell injected with X-rhodamine tubulin. Elapsed time in min/sec is in the upper right of each panel. The centrosome in this cell was positioned at the edge of the nucleus, so that half of the MTs emanating from the centrosome were visible beneath the nucleus. A MT was nucleated from the centrosome (small arrowhead) and grew radially out of the field of view (times 00:29–00:59). Another MT depolymerized from outside the field of view (large arrowhead) and was consumed by complete depolymerization all the way back to the centrosome (times 00:59–01: 59). Bar, 10 μm.
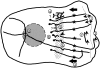
Figure 12
Model of MT arrangement and turnover in the lamella and lamellipodia of migrating newt lung epithelial cells. Thick lines represent MTs; dotted lines, the border between the lamella and lamellipodia; thin arrows, MT growth or shortening; arrowheads, sites of MT breakage; thick arrows, the direction of retrograde flow; dotted area, the putative zone of MT breakage at the base of the lamella; and circles, the centrosome. The numbers in the diagram refer to findings and hypotheses from this study. The cell is migrating to the right. 1, MTs in the lamella oriented perpendicular to the leading edge extend to the base of the lamellipodia, exhibit frequent and short dynamic instability, and show little net change in length. 2, Parallel MTs within the lamellipodia undergo catastrophe less often and exhibit net growth. 3, Parallel MTs and photoactivated marks on perpendicular MTs in the lamella (stars) move continuously towards the cell center at ∼0.4 μm/min. 4, F-actin (beaded lines) crosslinked to MTs is postulated to be moved rearward by myosin, which is bound to an unknown stationary structure (question mark) in the lamella. 5, MT breakage occurring at sites of local MT buckling. 6, Free minus ends formed by breakage are specifically capped (asterisks). 7, Treadmilling of MTs by net plus end growth and net minus end shortening. 8, <25% of all MTs in the cell are bound at their minus ends to the centrosome. 9, Cytoplasmic dynein bound to a membranous organelle (question mark) or other MT crosslinking proteins, are proposed to organize noncentrosomal MTs in the lamella.
Similar articles
- Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells.
Waterman-Storer CM, Salmon WC, Salmon ED. Waterman-Storer CM, et al. Mol Biol Cell. 2000 Jul;11(7):2471-83. doi: 10.1091/mbc.11.7.2471. Mol Biol Cell. 2000. PMID: 10888682 Free PMC article. - Converging populations of f-actin promote breakage of associated microtubules to spatially regulate microtubule turnover in migrating cells.
Gupton SL, Salmon WC, Waterman-Storer CM. Gupton SL, et al. Curr Biol. 2002 Nov 19;12(22):1891-9. doi: 10.1016/s0960-9822(02)01276-9. Curr Biol. 2002. PMID: 12445381 - Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms.
Waterman-Storer CM, Salmon ED. Waterman-Storer CM, et al. Curr Biol. 1998 Jul 2;8(14):798-806. doi: 10.1016/s0960-9822(98)70321-5. Curr Biol. 1998. PMID: 9663388 - [Dynamics and the life cycle of cell microtubules].
Vorob'ev IA, Grigor'ev IS. Vorob'ev IA, et al. Tsitol Genet. 2003 Mar-Apr;37(2):22-38. Tsitol Genet. 2003. PMID: 12774515 Review. Russian. - Generation of noncentrosomal microtubule arrays.
Bartolini F, Gundersen GG. Bartolini F, et al. J Cell Sci. 2006 Oct 15;119(Pt 20):4155-63. doi: 10.1242/jcs.03227. J Cell Sci. 2006. PMID: 17038542 Review.
Cited by
- Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse.
Hartzell CA, Jankowska KI, Burkhardt JK, Lewis RS. Hartzell CA, et al. Elife. 2016 Jul 21;5:e14850. doi: 10.7554/eLife.14850. Elife. 2016. PMID: 27440222 Free PMC article. - SMRT analysis of MTOC and nuclear positioning reveals the role of EB1 and LIC1 in single-cell polarization.
Hale CM, Chen WC, Khatau SB, Daniels BR, Lee JS, Wirtz D. Hale CM, et al. J Cell Sci. 2011 Dec 15;124(Pt 24):4267-85. doi: 10.1242/jcs.091231. Epub 2011 Dec 22. J Cell Sci. 2011. PMID: 22193958 Free PMC article. - Contribution of whole-cell optimization via cell body rolling to polarization of T cells.
Arkhipov SN, Maly IV. Arkhipov SN, et al. Phys Biol. 2006 Oct 3;3(3):209-19. doi: 10.1088/1478-3975/3/3/006. Phys Biol. 2006. PMID: 17021385 Free PMC article. - The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology.
Hahn I, Voelzmann A, Liew YT, Costa-Gomes B, Prokop A. Hahn I, et al. Neural Dev. 2019 Nov 9;14(1):11. doi: 10.1186/s13064-019-0134-0. Neural Dev. 2019. PMID: 31706327 Free PMC article. Review. - Structural microtubule cap: stability, catastrophe, rescue, and third state.
Jánosi IM, Chrétien D, Flyvbjerg H. Jánosi IM, et al. Biophys J. 2002 Sep;83(3):1317-30. doi: 10.1016/S0006-3495(02)73902-7. Biophys J. 2002. PMID: 12202357 Free PMC article.
References
- Abercrombie, M., G.A. Dunn, and J.P. Heath. 1976. Locomotion and contraction in nonmuscle cells. In Contractile systems in non-muscle tissues. S.V. Perry, editor. Amsterdam, North-Holland Publishers. 3–11.
- Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources