Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution - PubMed (original) (raw)
Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution
J K Burkhardt et al. J Cell Biol. 1997.
Abstract
Dynactin is a multisubunit complex that plays an accessory role in cytoplasmic dynein function. Overexpression in mammalian cells of one dynactin subunit, dynamitin, disrupts the complex, resulting in dissociation of cytoplasmic dynein from prometaphase kinetochores, with consequent perturbation of mitosis (Echeverri, C.J., B.M. Paschal, K.T. Vaughan, and R.B. Vallee. 1996. J. Cell Biol. 132:617-634). Based on these results, dynactin was proposed to play a role in linking cytoplasmic dynein to kinetochores and, potentially, to membrane organelles. The current study reports on the dynamitin interphase phenotype. In dynamitin-overexpressing cells, early endosomes (labeled with antitransferrin receptor), as well as late endosomes and lysosomes (labeled with anti-lysosome-associated membrane protein-1 [LAMP-1]), were redistributed to the cell periphery. This redistribution was disrupted by nocodazole, implicating an underlying plus end-directed microtubule motor activity. The Golgi stack, monitored using sialyltransferase, galactosyltransferase, and N-acetylglucosaminyltransferase I, was dramatically disrupted into scattered structures that colocalized with components of the intermediate compartment (ERGIC-53 and ERD-2). The disrupted Golgi elements were revealed by EM to represent short stacks similar to those formed by microtubule-depolymerizing agents. Golgi-to-ER traffic of stack markers induced by brefeldin A was not inhibited by dynamitin overexpression. Time-lapse observations of dynamitin-overexpressing cells recovering from brefeldin A treatment revealed that the scattered Golgi elements do not undergo microtubule-based transport as seen in control cells, but rather, remain stationary at or near their ER exit sites. These results indicate that dynactin is specifically required for ongoing centripetal movement of endocytic organelles and components of the intermediate compartment. Results similar to those of dynamitin overexpression were obtained by microinjection with antidynein intermediate chain antibody, consistent with a role for dynactin in mediating interactions of cytoplasmic dynein with specific membrane organelles. These results suggest that dynamitin plays a pivotal role in regulating organelle movement at the level of motor-cargo binding.
Figures
Figure 1
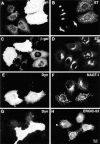
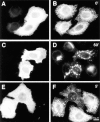
Disruption of exocytic organelles by dynamitin overexpression. HeLa cells were transiently transfected with dynamitin (A, B, E–H) or β-galactosidase (C and D), and the distribution of membrane markers in interphase cells was examined by double-label immunofluorescence microscopy. (A, C, E, and G) transfected cells labeled with antidynamitin (or anti-myc tag, E) or anti–β-galactosidase (C). (B and D) ST labeling of the _trans_-Golgi and TGN, showing that dynamitin overexpression disrupts the compact juxtanuclear Golgi complex. (F) NAGT-I labeling of the medial-Golgi shows disruption similar to ST. (H) ERGIC-53 labeling of the intermediate compartment between ER and _cis_-Golgi. Note that the juxtanuclear accumulation of ERGIC-53 is lost in the dynamitin-transfected cells, and that the normal punctate cytoplasmic fluorescence is somewhat exaggerated in dynamitin overexpressors.
Figure 2
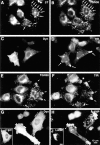
Disruption of endocytic organelles by dynamitin overexpression. HeLa cells were transfected with dynamitin (A–H) or β-galactosidase (I and J). Transfected cells are identified by labeling with antidynamitin (C and G), anti–β-galactosidase (I), or by the characteristic dynamitin-induced fragmentation of the Golgi (A and E). A and B show double labeling for ST and TGN38, respectively. In transfected cells, one pool of TGN38 colocalizes with ST (A and B, arrows), while another shifts to the periphery (B, arrowheads). (C–F) Effect of dynamitin on TfR distribution. TfR-labeled endosomes (D and F) are lost from the juxtanuclear recycling compartment, and in some cells, they accumulate at the tips of cell processes (arrows). This peripheral distribution is only infrequently observed in untransfected cells (broad arrow, F; see also Table I). Double labeling for TfR (F) and TGN38 (E) reveals that the two markers accumulate at overlapping peripheral sites (arrows). (H and J) The distribution of LAMP-1 in dynamitin overexpressors and control transfectants, respectively. Note the extreme shift of LAMP-positive late endosomes and lysosomes into the periphery. The cell elongation shown in H was sometimes observed in dynamitin-overexpressing cells, though in HeLa cells, this effect was infrequent and was not required for maximal organelle redistribution.
Figure 6
Dynamitin-mediated Golgi fragmentation resembles treatment with nocodazole. Cells transfected with dynamitin and incubated for ∼20 h to allow Golgi fragmentation were treated for 3 h with nocodazole (A and B), or treated and then allowed to recover in the absence of drug for an additional 3 h (C and D). Panels on the left show labeling for dynamitin, and those on the right for ST. After nocodazole treatment, the untransfected cell in B is indistinguishable from the dynamitin-transfected cell. Nocodazole has no effect on the dynamitin phenotype, but dynamitin overexpression prevents recovery from nocodazole. (D, arrow) An untransfected cell that has regenerated an intact Golgi complex upon nocodazole washout. (E–H) Golgi and IC/_cis_-Golgi network markers colocalize in dynamitin overexpressors. Cells transfected with dynamitin were labeled for the following pairs of marker proteins: ERGIC-53 (E) and ST (F); ERD-2 (G) and galactosyltransferase (H).
Figure 5
An intact microtubule cytoskeleton is required for dynamitin-induced lysosome redistribution. Cells transfected with dynamitin and incubated for ∼20 h to allow accumulation of lysosomes in the periphery were incubated for 3 h in the presence of nocodazole to depolymerize microtubules (A and B). Nocodazole was then washed out, and the cells were allowed to recover for 3 h in the absence of drug (C and D). A and C show labeling with antidynamitin; B and D show anti-LAMP label. Note that in the presence of nocodazole, lysosomes are randomly distributed in both transfected and untransfected cells, whereas the juxtanuclear concentration in untransfected cells and the peripheral concentration in transfectants are regenerated when microtubules are allowed to repolymerize.
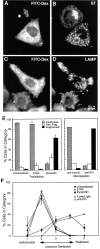
Figure 10
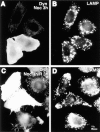
The effects of dynamitin overexpression are mimicked by microinjection of a function-blocking DIC antibody. HeLa cells were injected with a mixture of anti-DIC and fluorescent dextran, incubated for 4 h at 37°C, and then labeled for sialyltransferase (B) or LAMP (D). FITC-dextran-injected cells are shown in A and C (Note that the FITC signal was weak; the apparent punctate stain in A is an artifact of the strong LAMP fluorescence.) Anti-DIC disrupted the Golgi and peripheralized the lysosomes in a manner strikingly similar to dynamitin overexpression. (E and F) Quantitative comparison of the Golgi (E) and the lysosome (F) phenotypes resulting from microinjection and transfection. Cells were either injected with antibodies (E, right-hand portion; F, open symbols), or transfected with dynamitin or β-galactosidase (E, left-hand portion; F, closed symbols), and individual cells positive for the transfected proteins or for injected dextran were scored for the severity of the organelle redistribution, as described in Materials and Methods. For transfected cells, 50–60 cells were scored in each of three independent experiments. Data represent average scores ± 1 SD. For injected cells, data represent an average of 60–70 cells accumulated from two independent experiments. The effects of interfering with dynein and with dynactin were strikingly similar, and they were specific to these protein complexes.
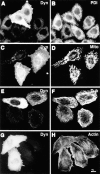
Figure 3
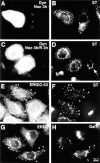
Cytoskeletal structures and certain organelles are not grossly affected by dynamitin overexpression in HeLa cells. HeLa cells transiently transfected with dynamitin were labeled with anti-dynamitin antibody (A, C, E, and G), and were double labeled with organelle markers. (B) Protein disulfide isomerase labeling the ER. (D) Antimitochondria antibody. (F) Anti–tubulin–labeling microtubules. (H) Bodipy phallicidin labeling filamentous actin. E and F show projections of confocal images to better show the microtubule arrays. The apparent microtubule staining pattern in E is an artifact resulting from the much stronger microtubule fluorescence.
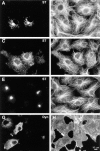
Figure 4
Effects of dynamitin overexpression on cytoskeletal structures in COS-7 cells do not correlate with Golgi disruption. (A–F) COS-7 cells were transiently transfected to coexpress VSV-G–tagged ST with either myc-tagged dynamitin (A–D), or β-galactosidase (E and F). More than 90% of overexpressing cells in cotransfected cultures were found to express both transfected constructs. Double- labeling of antitubulin (B, D, and F) with anti–VSV-G tag (A, C, and E) revealed clear Golgi disruptions in dynamitin-transfected cells with normal radial microtubule arrays (A and B), as well as in those showing less well- focused arrays (C and D). Golgi distribution and microtubule organization in β-galactosidase–transfected cells (E and F) were indistinguishable from those of control untransfected cells. As seen in HeLa cells, COS-7 cells overexpressing myc-tagged dynamitin alone and double-labeled with anti–myc tag (G) and rhodamine phalloidin (H) showed no apparent perturbations of the F-actin cytoskeleton.
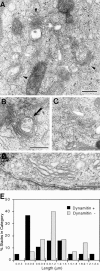
Figure 7
Dynamitin overexpression generates short Golgi stacks. Cells were cotransfected with dynamitin and GFP, and sorted into GFP-positive and GFP-negative pools. The GFP-positive pool was substantially enriched for dynamitin-positive cells, with 73% of cells expressing detectable dynamitin levels and 58% expressing high levels. The two cell populations were processed for EM, and the lengths of 20 randomly selected Golgi stacks in each sample were measured as described in Materials and Methods. (A–C) Representative short stacks found frequently in GFP/ dynamitin–positive cells. (D) Control Golgi stack. (E) Stack length distribution in the two cell populations shows a large increase in short stacks in the dynamitin/GFP–positive pool. The arrow in B denotes a multivesicular body seen in association with a short stack. Such structures were relatively common. Bars, 0.5 μm.
Figure 8
BFA treatment of dynamitin overexpressing cells. Transfected cells were treated with BFA for 20 min (A and B), or they were treated and allowed to recover in the absence of drug for 1 h (C and D) or for 10 min (E and F). Dynamitin overexpression did not prevent the redistribution of Golgi markers into the ER. The fragmented Golgi phenotype was generated at early times after BFA removal, without passage of marker proteins through a juxtanuclear Golgi complex.
Figure 9
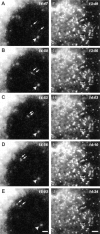
Time-lapse analysis of living COS-7 cells during recovery from BFA directly demonstrates dynamitin-induced inhibition of ER-to-Golgi transport. COS-7 cultures were transiently transfected with a GFP–NAGT-I fusion protein either alone (A–E) or with myc-tagged dynamitin (F–J). More than 90% of overexpressing cells in fixed, cotransfected cultures were found to express both transfected constructs. After a 20-min exposure to BFA, the drug was washed out and the GFP–NAGT-I fluorescence was recorded by time-lapse microscopy at 3- to 7-s intervals. (A–E) Cytoplasmic region beneath the nucleus with reforming Golgi complex at upper left corner. (F–J) Cytoplasmic region between nucleus at upper left corner and cell periphery at lower right corner. Control cells typically exhibited rapid centripetal movements of newly formed GFP–NAGT-I–positive vesicles (A–E, arrows; arrowhead indicates static vesicle for reference), leading to the reformation of a perinuclear Golgi complex (A–E, upper left corner). Similar labeled vesicles appeared throughout the cytoplasm of dynamitin-transfected cells, but no sustained movements were observed in any direction, as seen in F–J. Note partial alignments of vesicles in F–J, forming short linear arrays suggestive of interactions with microtubules. Bars, 1 μm.
Similar articles
- Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends.
Vaughan KT, Tynan SH, Faulkner NE, Echeverri CJ, Vallee RB. Vaughan KT, et al. J Cell Sci. 1999 May;112 ( Pt 10):1437-47. doi: 10.1242/jcs.112.10.1437. J Cell Sci. 1999. PMID: 10212138 - Dynein-dependent transport of the hantaan virus nucleocapsid protein to the endoplasmic reticulum-Golgi intermediate compartment.
Ramanathan HN, Chung DH, Plane SJ, Sztul E, Chu YK, Guttieri MC, McDowell M, Ali G, Jonsson CB. Ramanathan HN, et al. J Virol. 2007 Aug;81(16):8634-47. doi: 10.1128/JVI.00418-07. Epub 2007 May 30. J Virol. 2007. PMID: 17537852 Free PMC article. - Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes.
Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw CI, Willemsen R, Visser P, Grosveld F, Galjart N. Hoogenraad CC, et al. EMBO J. 2001 Aug 1;20(15):4041-54. doi: 10.1093/emboj/20.15.4041. EMBO J. 2001. PMID: 11483508 Free PMC article. - Role of microtubules in the organization of the Golgi complex.
Thyberg J, Moskalewski S. Thyberg J, et al. Exp Cell Res. 1999 Feb 1;246(2):263-79. doi: 10.1006/excr.1998.4326. Exp Cell Res. 1999. PMID: 9925741 Review. - The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex.
Burkhardt JK. Burkhardt JK. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):113-26. doi: 10.1016/s0167-4889(98)00052-4. Biochim Biophys Acta. 1998. PMID: 9714769 Review.
Cited by
- An organelle gatekeeper function for Caenorhabditis elegans UNC-16 (JIP3) at the axon initial segment.
Edwards SL, Yu SC, Hoover CM, Phillips BC, Richmond JE, Miller KG. Edwards SL, et al. Genetics. 2013 May;194(1):143-61. doi: 10.1534/genetics.112.147348. Genetics. 2013. PMID: 23633144 Free PMC article. - Exploitation of Cellular Cytoskeletons and Signaling Pathways for Cell Entry by Kaposi's Sarcoma-Associated Herpesvirus and the Closely Related Rhesus Rhadinovirus.
Zhang W, Gao SJ. Zhang W, et al. Pathogens. 2012 Dec;1(2):102-27. doi: 10.3390/pathogens1020102. Epub 2012 Oct 22. Pathogens. 2012. PMID: 23420076 Free PMC article. - Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi.
Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M, Tagaya M. Hirose H, et al. EMBO J. 2004 Mar 24;23(6):1267-78. doi: 10.1038/sj.emboj.7600135. Epub 2004 Mar 18. EMBO J. 2004. PMID: 15029241 Free PMC article. - Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules.
Xu A, Bellamy AR, Taylor JA. Xu A, et al. EMBO J. 2000 Dec 1;19(23):6465-74. doi: 10.1093/emboj/19.23.6465. EMBO J. 2000. PMID: 11101519 Free PMC article. - Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly.
Guo J, Yang Z, Song W, Chen Q, Wang F, Zhang Q, Zhu X. Guo J, et al. Mol Biol Cell. 2006 Feb;17(2):680-9. doi: 10.1091/mbc.e05-04-0360. Epub 2005 Nov 16. Mol Biol Cell. 2006. PMID: 16291865 Free PMC article.
References
- Allan V. Motor proteins: a dynamic duo. Curr Biology. 1996;6:630–633. - PubMed
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous