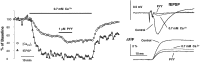Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry - PubMed (original) (raw)
Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry
J Qian et al. J Neurosci. 1997.
Abstract
Neuropeptide Y (NPY) agonists inhibit glutamate release by a presynaptic action at the CA3-CA1 synapse of rat hippocampus. We have examined the relationship between [Capre]t via presynaptic, voltage-dependent calcium channels (VDCCs), measured optically by using the fluorescent calcium indicator fura-2, and transmitter release, measured electrophysiologically. Activation of presynaptic NPY Y2 receptors reduced [Capre]t and thereby inhibited synaptic transmission. Multiple calcium channels are involved in synaptic transmission at this synapse. Activation of Y2 receptors inhibits N-type, P/Q-type, and unidentified presynaptic VDCCs. The inhibition of each of these calcium channel types contributes to the reduction of [Capre]t by Y2 receptors. Activation of adenosine receptors fully occluded the inhibition of presynaptic calcium influx by Y2 receptors but not the inhibition by GABAB receptors, suggesting a convergent action for Y2 and adenosine receptors, probably by coupling to the same G-protein.
Figures
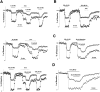
Fig. 1.
A nonlinear relationship exists between [Capre]t and fEPSP. A, Schematic diagram for loading calcium indicators into presynaptic terminals. Esterified (membrane-permeant) forms of fura-2 or furaptra were pressure-injected into stratum radiatum (SR), where they were taken up into CA3 axons, trapped by the action of esterases, and diffused into the remote presynaptic terminals onto CA1 neurons. Typical traces of the electrically recorded field EPSP (fEPSP), normalized transients of calcium indicator-related fluorescence (Fura), and their first derivative are shown under control conditions and during blocked synaptic transmission (CNQX+APV).B, Comparison between the peak of _ΔF/F_and the peak of the first derivative of ΔF/F.C, Semi-log plot of normalized synaptic transmission and [Capre]t versus extracellular calcium concentration, [Ca2+]o. [Capre]t was a logarithmic function of [Ca2+]o. D, Double-log plot of normalized fEPSP versus [Capre]t for each tested [Ca2+]o. The regression line has a slope of m = 4.2 (_r_2 = 0.99) for fura-2 measurement and 3.8 (_r_2 = 0.99) for furaptra, respectively.
Fig. 2.
The activation of presynaptic Y2receptors inhibits [Capre]t and fEPSP.A, Group data (n = 5) showing the time course of normalized [Capre]t and fEPSP during the application of 1 μ
m
PYY. Sample traces taken at control and during maximal action of PYY are shown in the_inset_. B, Summary data for eight experiments. Approximately 20% of [Capre]twas inhibited by application of 1 μ
m
PYY. The synaptic transmission was reduced by ∼60%. C, The presynaptic volley did not show a significant change during the application of PYY.D, Double-log plot of normalized fEPSP and [Capre]t during the peak action of PYY. Estimated power number for the inhibition of synaptic transmission by PYY was 3.9 ± 0.5 (n = 8). Similar power numbers were observed in this preparation for the action of PYY and the reduction of [Ca2+]o.
Fig. 3.
ω-CgTx GVIA does not abolish the effects of PYY.A, Group data (n = 6) showing the time course of normalized [Capre]t and fEPSP during the application of ω-CgTx GVIA (1 μ
m
) and PYY (1 μ
m
). Sample traces taken at control, after application of ω-CgTx GVIA, and during the peak effect of PYY are shown in the_inset_. B, Summary data for seven experiments. After preapplication of ω-CgTx GVIA, PYY could still inhibit [Capre]t but to a lesser extent (∼15% of control [Capre]t), whereas fEPSP was decreased to 80% of control. C, Double-log plot of normalized fEPSP and [Capre]t during application of ω-CgTx GVIA and ω-CgTx GVIA+PYY. Estimated power numbers were m = 3.5 ± 0.3 (n = 7) for the inhibition by ω-CgTx GVIA alone and m = 3.9 ± 0.4 (n = 7) for the combination of ω-CgTx GVIA and PYY. The peak responses of ω-CgTx GVIA and PYY were used to calculate the power numbers.
Fig. 4.
ω-Aga IVA and ω-CgTx MVIIC do not abolish the effects of PYY. A, Group data showing the time course of normalized [Capre]t and fEPSP during the action of ω-Aga IVA (500 n
m
) alone and together with 1 μ
m
PYY. Sample traces taken at control, after application of the calcium channel toxin, and during the peak effect of PYY are shown in the inset. B, Group data showing the time course of normalized [Capre]t and fEPSP during the action of PYY together with ω-CgTx MVIIC (1 μ
m
). Sample traces taken at control, after application of calcium channel toxins, and during peak effect of PYY are shown in the_inset_. C, Summary data for_A_. [Capre]t and synaptic transmission were reduced irreversibly by ∼34 and 80%, respectively, after application of ω-Aga IVA. PYY still inhibited [Capre]t by ∼15% of control [Capre]t after blockade of P/Q-type calcium channels, and synaptic transmission also was decreased further.D, Approximately 11% inhibition of [Capre]t was observed even after application of ω-CgTx MVIIC to stop synaptic transmission almost completely.
Fig. 5.
Activation of Y2 receptors occludes the inhibition of [Capre]t by adenosine.A, Time course of normalized [Capre]t and fEPSP illustrates the occlusion of inhibition of [Capre]t between activation of Y2 and adenosine (AD) receptors. The inhibition of [Capre]t by adenosine in the presence of PYY (1 μ
m
) was always smaller than that without activation of Y2 receptors, regardless of the concentrations of adenosine (5, 10, or 100 μ
m
).B, A saturating concentration of AD (100 μ
m
) completely abolished the inhibition of [Capre]t by PYY. C,D, Time course of normalized [Capre]t and fEPSP illustrates the partial occlusion between the activation of AD receptors and the activation of GABAB receptors by baclofen (50 μ
m
).
Fig. 6.
PYY inhibits the same percentage of [Capre]t in reduced [Ca2+]o. Shown is the time course of normalized [Capre]t and fEPSP in reduced [Ca2+]o (0.7 m
m
). Application of PYY still elicited a 23.5% inhibition of [Capre]t under these conditions. The_inset_ shows the sample traces taken under control conditions (2.5 m
m
[Ca2+]o), in the presence of 0.7 m
m
[Ca2+]o, and during the peak effect of PYY.
Similar articles
- Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus.
Silva AP, Carvalho AP, Carvalho CM, Malva JO. Silva AP, et al. Neuropharmacology. 2003 Feb;44(2):282-92. doi: 10.1016/s0028-3908(02)00382-9. Neuropharmacology. 2003. PMID: 12623227 - Presynaptic inhibition of synaptic transmission in the rat hippocampus by activation of muscarinic receptors: involvement of presynaptic calcium influx.
Qian J, Saggau P. Qian J, et al. Br J Pharmacol. 1997 Oct;122(3):511-9. doi: 10.1038/sj.bjp.0701400. Br J Pharmacol. 1997. PMID: 9351508 Free PMC article. - Mu-opioid and GABA(B) receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons.
Rusin KI, Moises HC. Rusin KI, et al. Neuroscience. 1998 Aug;85(3):939-56. doi: 10.1016/s0306-4522(97)00674-x. Neuroscience. 1998. PMID: 9639286 - Subtypes of receptors for neuropeptide Y: implications for the targeting of therapeutics.
Gehlert DR. Gehlert DR. Life Sci. 1994;55(8):551-62. doi: 10.1016/0024-3205(94)00481-1. Life Sci. 1994. PMID: 7913982 Review. - Neuropeptide Y-related peptides and their receptors--are the receptors potential therapeutic drug targets?
Wahlestedt C, Reis DJ. Wahlestedt C, et al. Annu Rev Pharmacol Toxicol. 1993;33:309-52. doi: 10.1146/annurev.pa.33.040193.001521. Annu Rev Pharmacol Toxicol. 1993. PMID: 8494343 Review. No abstract available.
Cited by
- Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors.
Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Peňa J, Navarro M, Kash TL, Thiele TE. Sparrow AM, et al. Neuropsychopharmacology. 2012 May;37(6):1409-21. doi: 10.1038/npp.2011.327. Epub 2012 Jan 4. Neuropsychopharmacology. 2012. PMID: 22218088 Free PMC article. - A peptide inhibitor of Tau-SH3 interactions ameliorates amyloid-β toxicity.
Rush T, Roth JR, Thompson SJ, Aldaher AR, Cochran JN, Roberson ED. Rush T, et al. Neurobiol Dis. 2020 Feb;134:104668. doi: 10.1016/j.nbd.2019.104668. Epub 2019 Nov 5. Neurobiol Dis. 2020. PMID: 31698056 Free PMC article. - Presynaptic Ca(2+) influx at a mouse central synapse with Ca(2+) channel subunit mutations.
Qian J, Noebels JL. Qian J, et al. J Neurosci. 2000 Jan 1;20(1):163-70. doi: 10.1523/JNEUROSCI.20-01-00163.2000. J Neurosci. 2000. PMID: 10627593 Free PMC article. - Presynaptic Ca2+ channels and neurotransmitter release at the terminal of a mouse cortical neuron.
Qian J, Noebels JL. Qian J, et al. J Neurosci. 2001 Jun 1;21(11):3721-8. doi: 10.1523/JNEUROSCI.21-11-03721.2001. J Neurosci. 2001. PMID: 11356859 Free PMC article. - Differential Effect of Neuropeptides on Excitatory Synaptic Transmission in Human Epileptic Hippocampus.
Ledri M, Sørensen AT, Madsen MG, Christiansen SH, Ledri LN, Cifra A, Bengzon J, Lindberg E, Pinborg LH, Jespersen B, Gøtzsche CR, Woldbye DP, Andersson M, Kokaia M. Ledri M, et al. J Neurosci. 2015 Jul 1;35(26):9622-31. doi: 10.1523/JNEUROSCI.3973-14.2015. J Neurosci. 2015. PMID: 26134645 Free PMC article.
References
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous