cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory - PubMed (original) (raw)
Comparative Study
cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory
R Lamprecht et al. J Neurosci. 1997.
Abstract
In conditioned taste aversion (CTA) organisms learn to avoid a taste if the first encounter with that taste is followed by transient poisoning. The neural mechanisms that subserve this robust and long-lasting association of taste and malaise have not yet been elucidated, but several brain areas have been implicated in the process, including the amygdala. In this study we investigated the role of amygdala in general, and the cAMP response element-binding protein (CREB) in the amygdala in particular, in CTA learning and memory. Toward that end, we combined antisense technology in vivo with behavioral, molecular, and histochemical analysis. Local microinjection of phosphorothioate-modified oligodeoxynucleotides (ODNs) antisense to CREB into the rat amygdala several hours before CTA training transiently reduced the level of CREB protein during training and impaired CTA memory when tested 3-5 d later. In comparison, sense ODNs had no effect on memory. The effect of antisense was not attributable to differential tissue damage and was site-specific. CREB antisense in the amygdala had no effect on retrieval of CTA memory once it had been formed, and did not affect short-term CTA memory. We propose that the amygdala, specifically the central nucleus, is required for the establishment of long-term CTA memory in the behaving rat; that the process involves long-term changes, subserved by CRE-regulated gene expression, in amygdala neurons; and that the amygdala may retain some CTA-relevant information over time rather than merely modulating the gustatory trace during acquisition of CTA.
Figures
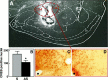
Fig. 1.
Sphere of diffusion of microinjected CREB antisense ODNs into the amygdala (A) and reduction in CREB-positive nuclei by CREB antisense (B–D). A, FITC-labeled CREB antisense was microinjected, and the fluorescence image recorded as detailed in Materials and Methods. CeM, Central amygdaloid nucleus, medial division; CeL, central amygdaloid nucleus, lateral division; BMA, basomedial amygdaloid nucleus, anterior part; BLA, basolateral amygdaloid nucleus, anterior part; Pir, piriform cortex. The microinjection cannula track and the lesion induced by the tip of the cannula are seen within the sphere of FITC diffusion. The schematic anatomical map is adapted from that of Paxinos and Watson (1986).B, The number of CREB-positive nuclei in the amygdala of CREB antisense (AS) versus sense (S) microinjected rats. In both groups CREB antisense was microinjected into the amygdala 14 hr before CTA training; n = 4 in each group. The_asterisk_ indicates significance for pair comparison in which a Scheffe contrast test was used with an α of 0.05. C, D, Immunohistochemistry of CREB using CREB antibodies in CREB sense (C) and antisense (D) microinjected rats. The selected fields were taken from the region of the CeL, indicated in A; dot, edge of the microinjection cannula-induced lesion.
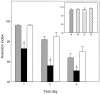
Fig. 2.
Impairment of CTA by ODNs antisense to CREB in the amygdala. Aversion indices are plotted versus the test day. In each test day, the left (shaded)bar depicts the aversion index of normal CTA control animals (n = 23); the center(solid) bar depicts the aversion index of animals locally microinjected into the amygdala with antisense ODNs (n = 16); and the right(open) bar shows the index of animals receiving sense ODNs (n = 11). All microinjections into the amygdala were performed 14 hr before CTA training, as detailed in Materials and Methods. Inset, Spatial and temporal specificity of the antisense effect. The _shaded bar_depicts the result for normal controls; the _hatched bars_show the results for rats microinjected with antisense.A, Normal CTA animals; B, antisense microinjected 14 hr before training 2 mm above the stereotaxic coordinates used for injection into the amygdala; C, antisense microinjected into the amygdala 14 hr before the first memory test, i.e., 36 hr after the completion of training; D, antisense microinjected into the amygdala 7 d before CTA training;n = 6 animals in each group.Asterisks indicate significance for pair comparison in which a Scheffe contrast test was used with an α of 0.05.
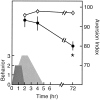
Fig. 3.
CREB antisense in the amygdala selectively impairs long- but not short-term memory. Aversion indices are plotted versus the time of test after CTA training. Closed circles, Rats microinjected with CREB antisense ODNs 14 hr before CTA training;open circles, control animals injected with saline instead of CREB antisense. The figure also depicts the time windows of LOB behavior (dark gray) and rearing (light gray), i.e., measures of the on-line effect of the malaise inducing-agent as a function of the time after LiCl injection intraperitoneally. For further details see Results. The_asterisk_ indicates significance for pair comparison in which a Scheffe contrast test was used with an α of 0.05.
Similar articles
- Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory.
Lamprecht R, Dudai Y. Lamprecht R, et al. Learn Mem. 1996 Jul-Aug;3(1):31-41. doi: 10.1101/lm.3.1.31. Learn Mem. 1996. PMID: 10456074 - Region-specific involvement of BDNF secretion and synthesis in conditioned taste aversion memory formation.
Ma L, Wang DD, Zhang TY, Yu H, Wang Y, Huang SH, Lee FS, Chen ZY. Ma L, et al. J Neurosci. 2011 Feb 9;31(6):2079-90. doi: 10.1523/JNEUROSCI.5348-10.2011. J Neurosci. 2011. PMID: 21307245 Free PMC article. - Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats.
Gallo M, Roldan G, Bures J. Gallo M, et al. Behav Brain Res. 1992 Nov 30;52(1):91-7. doi: 10.1016/s0166-4328(05)80328-6. Behav Brain Res. 1992. PMID: 1335264 - Conditioned taste aversion as a learning and memory paradigm.
Welzl H, D'Adamo P, Lipp HP. Welzl H, et al. Behav Brain Res. 2001 Nov 1;125(1-2):205-13. doi: 10.1016/s0166-4328(01)00302-3. Behav Brain Res. 2001. PMID: 11682112 Review. - Inducible repression of CREB function disrupts amygdala-dependent memory.
Josselyn SA, Kida S, Silva AJ. Josselyn SA, et al. Neurobiol Learn Mem. 2004 Sep;82(2):159-63. doi: 10.1016/j.nlm.2004.05.008. Neurobiol Learn Mem. 2004. PMID: 15341801 Review.
Cited by
- CREB: a multifaceted regulator of neuronal plasticity and protection.
Sakamoto K, Karelina K, Obrietan K. Sakamoto K, et al. J Neurochem. 2011 Jan;116(1):1-9. doi: 10.1111/j.1471-4159.2010.07080.x. Epub 2010 Dec 2. J Neurochem. 2011. PMID: 21044077 Free PMC article. Review. - Maged1 co-interacting with CREB through a hexapeptide repeat domain regulates learning and memory in mice.
Yang J, Lai B, Xu A, Liu Y, Li X, Zhao Y, Li W, Ji M, Hu G, Gao X, Gao J. Yang J, et al. Mol Neurobiol. 2015 Feb;51(1):8-18. doi: 10.1007/s12035-014-8677-x. Epub 2014 Apr 4. Mol Neurobiol. 2015. PMID: 24700102 - A neural mechanism for learning from delayed postingestive feedback.
Zimmerman CA, Bolkan SS, Pan-Vazquez A, Wu B, Keppler EF, Meares-Garcia JB, Guthman EM, Fetcho RN, McMannon B, Lee J, Hoag AT, Lynch LA, Janarthanan SR, López Luna JF, Bondy AG, Falkner AL, Wang SS, Witten IB. Zimmerman CA, et al. bioRxiv [Preprint]. 2024 Sep 19:2023.10.06.561214. doi: 10.1101/2023.10.06.561214. bioRxiv. 2024. PMID: 37873112 Free PMC article. Preprint. - Anterior Commissure Regulates Neuronal Activity of Amygdalae and Influences Locomotor Activity, Social Interaction and Fear Memory in Mice.
Hsu TT, Huang TN, Hsueh YP. Hsu TT, et al. Front Mol Neurosci. 2020 Mar 31;13:47. doi: 10.3389/fnmol.2020.00047. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32296306 Free PMC article. - Histamine in the basolateral amygdala promotes inhibitory avoidance learning independently of hippocampus.
Benetti F, Furini CR, de Carvalho Myskiw J, Provensi G, Passani MB, Baldi E, Bucherelli C, Munari L, Izquierdo I, Blandina P. Benetti F, et al. Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2536-42. doi: 10.1073/pnas.1506109112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918368 Free PMC article.
References
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. - PubMed
- Bermudez-Rattoni F, Grijalva CV, Kiefer SW, Garcia J. Flavor-illness aversions: the role of the amygdala in the acquisition of taste-potentiated odor aversions. Physiol Behav. 1986;38:503–508. - PubMed
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. - PubMed
- Bures J, Buresova O, Kriveanek J. Brain and behavior: paradigms for research on neuronal mechanisms. Wiley; New York: 1988.
- Burnette WW. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical