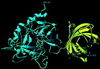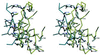Structure of the thrombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug - PubMed (original) (raw)
Comparative Study
Structure of the thrombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug
P Fuentes-Prior et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Triabin, a 142-residue protein from the saliva of the blood-sucking triatomine bug Triatoma pallidipennis, is a potent and selective thrombin inhibitor. Its stoichiometric complex with bovine alpha-thrombin was crystallized, and its crystal structure was solved by Patterson search methods and refined at 2.6-A resolution to an R value of 0.184. The analysis revealed that triabin is a compact one-domain molecule essentially consisting of an eight-stranded beta-barrel. The eight strands A to H are arranged in the order A-C-B-D-E-F-G-H, with the first four strands exhibiting a hitherto unobserved up-up-down-down topology. Except for the B-C inversion, the triabin fold exhibits the regular up-and-down topology of lipocalins. In contrast to the typical ligand-binding lipocalins, however, the triabin barrel encloses a hydrophobic core intersected by a unique salt-bridge cluster. Triabin interacts with thrombin exclusively via its fibrinogen-recognition exosite. Surprisingly, most of the interface interactions are hydrophobic. A prominent exception represents thrombin's Arg-77A side chain, which extends into a hydrophobic triabin pocket forming partially buried salt bridges with Glu-128 and Asp-135 of the inhibitor. The fully accessible active site of thrombin in this complex is in agreement with its retained hydrolytic activity toward small chromogenic substrates. Impairment of thrombin's fibrinogen converting activity or of its thrombomodulin-mediated protein C activation capacity upon triabin binding is explained by usage of overlapping interaction sites of fibrinogen, thrombomodulin, and triabin on thrombin. These data demonstrate that triabin inhibits thrombin via a novel and unique mechanism that might be of interest in the context of potential therapeutic applications.
Figures
Figure 1
Ribbon plot of the complex formed between bovine α-thrombin (blue) and triabin (yellow). The proteinase is shown in its “standard” orientation—i.e., with the active-site cleft facing the viewer and binding peptide substrates running from left to right. Side chains of the catalytic triad residues of thrombin are explicitly shown, as well as the important interface residues Arg-77A (thrombin) and Asp-135I (triabin). The figure was made with
setor
(8).
Figure 2
Stereoview of the triabin ribbon. The eight strands A to H forming the β- barrel are shown as yellow arrows, the N- and the C-terminal surface helices are shown as green helical ribbons, and the connecting loops are shown as green ropes. A few aromatic side chains, as well as the polar residues involved in the internal salt-bridge cluster, are shown with all nonhydrogen atoms. The view is, similar as in Fig. 1, approximately along the barrel axis of triabin—i.e., through the more narrow barrel opening (front) toward the wider opening of the calyx (back). The figure was made with
setor
(8).
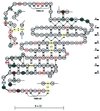
Figure 3
Schematic representation of the polypeptide chain arrangement of triabin. Acidic, basic, and cysteine residues are emphasized with red, blue, and yellow circles, respectively. Disulfide bridges are shown explicitly, and inter-main chain hydrogen bonds are represented as dotted lines. Residues involved in interactions with thrombin are shadowed in dark gray if in contact with Tyr-76 and/or Arg-77A, and in light gray otherwise. The shear number S equals the residue offset upon surrounding the barrel.
Figure 4
Closed-up stereo-view of the interaction interface between bovine thrombin and triabin. The contacting segments of thrombin (blue) and triabin (yellow) are shown as α-carbon traces, and only the more important side chains are given with all atoms. Water molecules are omitted for the sake of simplicity. Orientation is similar to that seen in Figs. 1 and 2. The figure was made with
setor
(8).
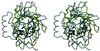
Figure 5
Stereoview of the Cα structures of triabin (yellow) and of the bilin binding protein (blue) after optimal least-squares fit of the eight strands. The residue numbering is that for triabin. The bound biliverdin pigment (gray) is presented to indicate the cavity in the ligand binding lipocalins. The side chains of residues Trp-25I and Arg-129I and of the triabin specific residue Asp-135I are given in full length. The figure made with
setor
(8).
Figure 6
Structure-based alignment of the amino acid sequences of triabin and of the lipocalins of known three-dimensional structure: retinol binding protein (RETB_HUMAN; refs. and 35), odorant binding protein (OBP_BOVIN; ref. 37), major urinary protein (MUP1_MOUSE; ref. 38), insecticyanin (ICYA_MANSE; ref. 39), retinoic acid binding protein (ERBP_RAT; ref. 40), and bilin binding protein (BBP_PIER; ref. 29). The three major structurally conserved regions (SCRs 1–3) are indicated. Numbers refer to the triabin sequence. Topologically equivalent residues between triabin and retinol binding protein are indicated by asterisks. Note that they correspond to the “reversed” sequence for residues 29I to 64I. The aligned sequences were formatted using the program
alscript
(41).
Similar articles
- An Insight into the Triabin Protein Family of American Hematophagous Reduviids: Functional, Structural and Phylogenetic Analysis.
Hernández-Vargas MJ, Santibáñez-López CE, Corzo G. Hernández-Vargas MJ, et al. Toxins (Basel). 2016 Feb 15;8(2):44. doi: 10.3390/toxins8020044. Toxins (Basel). 2016. PMID: 26891325 Free PMC article. - Triabin, a highly potent exosite inhibitor of thrombin.
Noeske-Jungblut C, Haendler B, Donner P, Alagon A, Possani L, Schleuning WD. Noeske-Jungblut C, et al. J Biol Chem. 1995 Dec 1;270(48):28629-34. doi: 10.1074/jbc.270.48.28629. J Biol Chem. 1995. PMID: 7499380 - A player of many parts: the spotlight falls on thrombin's structure.
Stubbs MT, Bode W. Stubbs MT, et al. Thromb Res. 1993 Jan 1;69(1):1-58. doi: 10.1016/0049-3848(93)90002-6. Thromb Res. 1993. PMID: 8465268 Review. - Nitrophorins and related antihemostatic lipocalins from Rhodnius prolixus and other blood-sucking arthropods.
Montfort WR, Weichsel A, Andersen JF. Montfort WR, et al. Biochim Biophys Acta. 2000 Oct 18;1482(1-2):110-8. doi: 10.1016/s0167-4838(00)00165-5. Biochim Biophys Acta. 2000. PMID: 11058753 Review.
Cited by
- Evolutionary families of peptidase inhibitors.
Rawlings ND, Tolle DP, Barrett AJ. Rawlings ND, et al. Biochem J. 2004 Mar 15;378(Pt 3):705-16. doi: 10.1042/BJ20031825. Biochem J. 2004. PMID: 14705960 Free PMC article. Review. - Protease signaling in animal and plant-regulated cell death.
Salvesen GS, Hempel A, Coll NS. Salvesen GS, et al. FEBS J. 2016 Jul;283(14):2577-98. doi: 10.1111/febs.13616. Epub 2015 Dec 31. FEBS J. 2016. PMID: 26648190 Free PMC article. Review. - An insight into the sialome of the blood-sucking bug Triatoma infestans, a vector of Chagas' disease.
Assumpção TC, Francischetti IM, Andersen JF, Schwarz A, Santana JM, Ribeiro JM. Assumpção TC, et al. Insect Biochem Mol Biol. 2008 Feb;38(2):213-32. doi: 10.1016/j.ibmb.2007.11.001. Epub 2007 Nov 17. Insect Biochem Mol Biol. 2008. PMID: 18207082 Free PMC article. - An Insight into the Triabin Protein Family of American Hematophagous Reduviids: Functional, Structural and Phylogenetic Analysis.
Hernández-Vargas MJ, Santibáñez-López CE, Corzo G. Hernández-Vargas MJ, et al. Toxins (Basel). 2016 Feb 15;8(2):44. doi: 10.3390/toxins8020044. Toxins (Basel). 2016. PMID: 26891325 Free PMC article. - Crystal structure of the human alpha-thrombin-haemadin complex: an exosite II-binding inhibitor.
Richardson JL, Kröger B, Hoeffken W, Sadler JE, Pereira P, Huber R, Bode W, Fuentes-Prior P. Richardson JL, et al. EMBO J. 2000 Nov 1;19(21):5650-60. doi: 10.1093/emboj/19.21.5650. EMBO J. 2000. PMID: 11060016 Free PMC article.
References
- Davie E W, Fujikawa K, Kisiel W. Biochemistry. 1991;30:10363–10370. - PubMed
- Stubbs M T, Bode W. Trends Biochem Sci. 1995;20:23–28. - PubMed
- Stubbs M T, Oschkinat H, Mayr I, Huber R, Angliker H, Stone S R, Bode W. Eur J Biochem. 1992;206:187–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources