The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport - PubMed (original) (raw)
The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport
C M Waterman-Storer et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Fast axonal transport is characterized by the bidirectional, microtubule-based movement of membranous organelles. Cytoplasmic dynein is necessary but not sufficient for retrograde transport directed from the synapse to the cell body. Dynactin is a heteromultimeric protein complex, enriched in neurons, that binds to both microtubules and cytoplasmic dynein. To determine whether dynactin is required for retrograde axonal transport, we examined the effects of anti-dynactin antibodies on organelle transport in extruded axoplasm. Treatment of axoplasm with antibodies to the p150(Glued) subunit of dynactin resulted in a significant decrease in the velocity of microtubule-based organelle transport, with many organelles bound along microtubules. We examined the molecular mechanism of the observed inhibition of motility, and we demonstrated that antibodies to p150(Glued) disrupted the binding of cytoplasmic dynein to dynactin and also inhibited the association of cytoplasmic dynein with organelles. In contrast, the anti-p150(Glued) antibodies had no effect on the binding of dynactin to microtubules nor on cytoplasmic dynein-driven microtubule gliding. These results indicate that the interaction between cytoplasmic dynein and the dynactin complex is required for the axonal transport of membrane-bound vesicles and support the hypothesis that dynactin may function as a link between the organelle, the microtubule, and cytoplasmic dynein during vesicle transport.
Figures
Figure 1
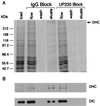
Characterization of the squid dynactin complex. (A) SDS/PAGE and immunoblot analysis of squid optic lobe cytosol (first lane), axoplasm (second lane), and a bovine brain fraction highly enriched in dynactin (third lane), stained for total protein, and probed with the anti-p150Glued antibodies UP235 and UP236 and with anti-centractin antibodies UP384 and UP385. (B) Squid optic lobe cytosol was sedimented on a 5–25% linear sucrose gradient, and gradient fractions were analyzed by SDS/PAGE and immunoblot with antibodies to p150Glued (Upper) and to centractin (Lower). The arrow indicates 20 S. (C) Anti-p150Glued antibody UP236 was used to immunoprecipitate dynactin from squid optic lobe cytosol. The immunoprecipitate was resolved by 8% SDS/PAGE and stained for total protein with Coomassie blue (lane 1). The band at 55 kDa is the IgG heavy chain, and the band at 66 kDa is BSA. Immunoblot analysis of the immunoprecipitate with anti-p150Glued antibody UP235 (lane 2) and anti-centractin antibody UP384 (lane 3). (D) Axoplasmic organelles were resolved by SDS/PAGE on a 10% gel and stained for total protein (lane 1). Immunoblotting was performed with anti-p150Glued antibody UP235 (lane 2) and anti-centractin antibody UP384 (lane 3).
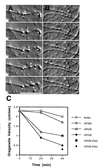
Figure 2
Effects of antibodies on organelle motility along individual microtubules. VEC-DIC micrographs of axoplasm taken at 1S intervals following a ≈45-min incubation in 0.8 mg/ml anti-p150Glued antibody UP-235 (A) or after ≈45 min in motility buffer (B). (A) Organelle at white carat is moving at 0.0 μm/sec; organelle at white star is moving at 0.3 μm/sec; organelle at white dot is moving at 0.3 μm/sec in the opposite direction along the same microtubule. (B) Organelle at black star moving at 2.0 μm/sec. Bar = 10 μm. (C) Average velocities of organelle motility along individual microtubules were determined at 20 and 40 min. Affinity-purified antibodies to p150Glued (UP235 or UP236) or control antibodies (UP385) were at 0.8 mg/ml; Fab fragments of affinity-purified anti-p150Glued antibodies [UP235 (Fab) or UP236 (Fab)] were at 0.5 mg/ml. Average velocities for organelle motility [presented as mean (in μm/sec) +/− SEM] are as follows: buffer, 20 min: 2.3 ± 0.2, n = 40, 40 min: 2.0 ± 0.2, n = 36; UP385, 20 min: 2.2 ± 0.2, n = 20, 40 min: 1.7 ± 0.3, n = 22; UP236, 20 min: 1.2 ± 0.3, n = 10, 40 min: 1.0 ± 0.2, n = 10, UP235, 20 min: 0.9 ± 0.2, n = 36, 40 min: 0.5 ± 0.1, n = 18, UP236 Fab fragments, 40 min: 1.0 ± 0.5, n = 13, UP235 Fab fragments, 40 min: 0.3 ± 0.1, n = 6.
Figure 3
Effects of anti-p150Glued antibodies on the dynein–dynactin interaction. Optic lobe cytosol was loaded onto p150Glued affinity columns that were preadsorbed with either preimmune serum or UP235 serum. The columns were eluted with 0.5 M NaCl in motility buffer. The resulting load, flowthrough, wash, and eluate fractions were analyzed by SDS/PAGE stained for total protein with Coomassie blue (A) and by immunoblotting (B) using antibodies to the dynein heavy chain (DHC, a generous gift of David Asai) or the dynein intermediate chain (DIC, a generous gift of Walter Steffen).
Figure 4
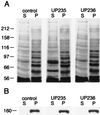
Effects of anti-p150Glued antibodies on the microtubule–dynactin interaction. Optic lobe cytosol was incubated for 45 min in the absence (control lanes) or in the presence of 1 mg/ml of the affinity-purified anti-p150Glued antibody UP235 or UP236. Microtubules were then assembled in the cytosol and isolated by centrifugation. The postmicrotubule supernatants (S) and microtubule pellets (P) were then analyzed by 8% SDS/PAGE and Coomassie blue staining for total protein (A) followed by immunoblotting with an antibody to p150Glued (B). Molecular mass in kDa is indicated on the left.
Figure 5
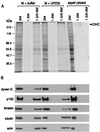
Effects of anti-p150Glued antibodies on the dynein–organelle interaction. A membrane-enriched fraction from squid was divided in half and either resuspended in motility buffer alone (M + buffer) or in buffer with the anti-p150Glued antibody UP235 at 0.8 mg/ml final concentration (M + UP235). Squid cytosol was used as a control for these experiments. The samples were fractionated on step gradients, and samples from the 1.5/0.6-M interface (vesicular) and the 1.5- and 2-M (soluble) fractions were analyzed by SDS/PAGE (stained with Coomassie blue for total protein, A) and by immunoblot (B) with antibodies to cytoplasmic dynein, dynactin, kinesin, tubulin, and actin. (Anti-dynein intermediate chain antibody was provided by Walter Steffen, anti-kinesin antibody was provided by B. Schnapp, anti-tubulin antibody was from Sigma, and anti-actin antibody was from Boehringer Mannheim.) The gels and blots were analyzed by densitometry; when the results are normalized for equal loading per lane, there was no significant change detected in the amount of dynactin or kinesin associated with membranes, whereas the reduction in the amount of dynein associated with membranes was found to be significant.
Similar articles
- Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport.
Martin M, Iyadurai SJ, Gassman A, Gindhart JG Jr, Hays TS, Saxton WM. Martin M, et al. Mol Biol Cell. 1999 Nov;10(11):3717-28. doi: 10.1091/mbc.10.11.3717. Mol Biol Cell. 1999. PMID: 10564267 Free PMC article. - Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport.
Dillman JF 3rd, Dabney LP, Karki S, Paschal BM, Holzbaur EL, Pfister KK. Dillman JF 3rd, et al. J Neurosci. 1996 Nov 1;16(21):6742-52. doi: 10.1523/JNEUROSCI.16-21-06742.1996. J Neurosci. 1996. PMID: 8824315 Free PMC article. - Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids.
Muresan V, Stankewich MC, Steffen W, Morrow JS, Holzbaur EL, Schnapp BJ. Muresan V, et al. Mol Cell. 2001 Jan;7(1):173-83. doi: 10.1016/s1097-2765(01)00165-4. Mol Cell. 2001. PMID: 11172722 - The role of the dynactin complex in intracellular motility.
Holleran EA, Karki S, Holzbaur EL. Holleran EA, et al. Int Rev Cytol. 1998;182:69-109. doi: 10.1016/s0074-7696(08)62168-3. Int Rev Cytol. 1998. PMID: 9522459 Review. - Cytoplasmic dynein and microtubule transport in the axon: the action connection.
Pfister KK. Pfister KK. Mol Neurobiol. 1999 Oct-Dec;20(2-3):81-91. doi: 10.1007/BF02742435. Mol Neurobiol. 1999. PMID: 10966115 Review.
Cited by
- Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients.
Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T. Sadakata T, et al. J Clin Invest. 2007 Apr;117(4):931-43. doi: 10.1172/JCI29031. Epub 2007 Mar 22. J Clin Invest. 2007. PMID: 17380209 Free PMC article. - Mutations in Caenorhabditis elegans cytoplasmic dynein components reveal specificity of neuronal retrograde cargo.
Koushika SP, Schaefer AM, Vincent R, Willis JH, Bowerman B, Nonet ML. Koushika SP, et al. J Neurosci. 2004 Apr 21;24(16):3907-16. doi: 10.1523/JNEUROSCI.5039-03.2004. J Neurosci. 2004. PMID: 15102906 Free PMC article. - Retrograde axonal transport and motor neuron disease.
Ström AL, Gal J, Shi P, Kasarskis EJ, Hayward LJ, Zhu H. Ström AL, et al. J Neurochem. 2008 Jul;106(2):495-505. doi: 10.1111/j.1471-4159.2008.05393.x. Epub 2008 Apr 1. J Neurochem. 2008. PMID: 18384644 Free PMC article. Review. - Dynein/dynactin is necessary for anterograde transport of Mbp mRNA in oligodendrocytes and for myelination in vivo.
Herbert AL, Fu MM, Drerup CM, Gray RS, Harty BL, Ackerman SD, O'Reilly-Pol T, Johnson SL, Nechiporuk AV, Barres BA, Monk KR. Herbert AL, et al. Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):E9153-E9162. doi: 10.1073/pnas.1711088114. Epub 2017 Oct 12. Proc Natl Acad Sci U S A. 2017. PMID: 29073112 Free PMC article. - Differential regulation of polarized synaptic vesicle trafficking and synapse stability in neural circuit rewiring in Caenorhabditis elegans.
Kurup N, Yan D, Kono K, Jin Y. Kurup N, et al. PLoS Genet. 2017 Jun 21;13(6):e1006844. doi: 10.1371/journal.pgen.1006844. eCollection 2017 Jun. PLoS Genet. 2017. PMID: 28636662 Free PMC article.
References
- Schnapp B J, Vale R D, Sheetz M P, Reese T S. Cell. 1985;40:455–462. - PubMed
- Vale R D, Schnapp B J, Reese T S, Sheetz M P. Cell. 1985;40:449–454. - PubMed
- Hirokawa N. Neurosci Res. 1993;18:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources