The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin - PubMed (original) (raw)
The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin
D N Hebert et al. J Cell Biol. 1997.
Abstract
Calnexin and calreticulin are homologous molecular chaperones that promote proper folding, oligomeric assembly, and quality control of newly synthesized glycoproteins in the endoplasmic reticulum (ER). Both are lectins that bind to substrate glycoproteins that have monoglucosylated N-linked oligosaccharides. Their binding to newly translated influenza virus hemagglutinin (HA), and various mutants thereof, was analyzed in microsomes after in vitro translation and expression in live CHO cells. A large fraction of the HA molecules was found to occur in ternary HA- calnexin-calreticulin complexes. In contrast to calnexin, calreticulin was found to bind primarily to early folding intermediates. Analysis of HA mutants with different numbers and locations of N-linked glycans showed that although the two chaperones share the same carbohydrate specificity, they display distinct binding properties; calreticulin binding depends on the oligosaccharides in the more rapidly folding top/hinge domain of HA whereas calnexin is less discriminating. Calnexin's binding was reduced if the HA was expressed as a soluble anchor-free protein rather than membrane bound. When the co- and posttranslational folding and trimerization of glycosylation mutants was analyzed, it was observed that removal of stem domain glycans caused accelerated folding whereas removal of the top domain glycans (especially the oligosaccharide attached to Asn81) inhibited folding. In summary, the data established that individual N-linked glycans in HA have distinct roles in calnexin/calreticulin binding and in co- and posttranslational folding.
Figures
Figure 1
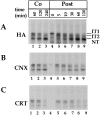
Calnexin and calreticulin differ in their association with HA in microsomes. 35S-HA was translated in vitro in the presence of canine pancreas microsomes at 32°C under oxidizing conditions (A–C, Co, lanes 1–3), or under reducing conditions. At time zero (lane 4), GSSG was added to initiate posttranslational oxidation (lanes 5–9). Alkylated samples were divided into three fractions for immunoprecipitation with anti-HA (A, HA), anti-calnexin (B, CNX), or anti-calreticulin antibodies (C, CRT), and then subjected to nonreducing SDS-PAGE and visualized by autoradiography. Partially oxidized intermediates IT1 and IT2 and the fully oxidized native HA (NT) were resolved.
Figure 2
Cotranslational association of HA with calnexin and calreticulin. Influenza-infected CHO cells were pulsed for 1.5 min with [35S]methionine/cysteine, and then the lysate was divided into three, immunoprecipitated, and analyzed by 2-D SDS-PAGE (nonreducing in the first dimension and reducing in the second dimension). (A) HA immunoprecipitated with an antibody against the NH2 terminus of HA (α-NHA). (B) HA immunoprecipitated sequentially with antibody against calnexin and antibody against HA NH2 terminus (α-CNX/α-NHA). (C) HA immunoprecipitated sequentially with antibody against calreticulin and antibody against HA NH2 terminus (α-CRT/α-NHA). The circles above the 2-D gels denote molecular markers of 66 and 45 kD. Exposure time for B and C was five times longer than A.
Figure 9
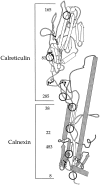
Calnexin and calreticulin binding regions on HA. The structure of the ectodomain of HA, modified from Wiley and co-workers (Wilson et al., 1981) is depicted with N-linked glycans and the disulfide bonds designated by the circles and the ball-and-sticks, respectively. The large disulfide loop, Cys 14-466, is represented by the unfilled ball-and-sticks. The numbers refer to the Asn residue that contain the N-linked glycosylations.
Figure 3
HA is found in calnexin–calreticulin ternary complexes. The presence of ternary calnexin–calreticulin–HA complexes was analyzed using double immunoprecipitations. (A) HA was translated as in Fig. 1 for 1 h at 32°C under oxidizing conditions. HA was first immunoprecipitated with anti-HA (_1_°, HA, lanes 1 and 2), -calnexin (_1_°, CNX, lanes 3–5), and -calreticulin (_1_°, CRT, lanes 6–8) antisera. The supernatants were cleared with protein A and reprecipitated with the indicated antisera (2°). (B) Bands from the autoradiogram in A were quantified by a digital densitometer and plotted as the fraction of total HA (IT1 + IT2 + NT from lane 1).
Figure 4
Folding, oligomerization, and calnexin/calreticulin binding of single glycan deletion mutants. (A) Wild-type and single glycosylation mutants of HA were translated under oxidizing conditions in the presence of canine pancreas microsomes at 32°C for 1 h. At the indicated times, samples were removed, alkylated, lysed, and immunoprecipitated with anti-HA antibodies. HA was resolved by nonreducing SDS-PAGE and autoradiography. (B) Translations were performed as above with oxidation carried out for 8 h at 32°C. The alkylated HA was immunoprecipitated with anti-trimer specific HA antibodies and resolved by reducing SDS-PAGE and autoradiography. (C) Translation of 35S-labeled HA were performed as in A for 1 h at 32°C. After alkylation, HA was immunoprecipitated with anti-HA, -calnexin, and -calreticulin antibodies. HA was resolved upon nonreducing and reducing SDS-PAGE and autoradiography. The fraction HA coprecipitating with calnexin or calreticulin was calculated as the amount of anti-calnexin or -calreticulin precipitable HA divided by the total HA precipitated with anti-HA antibodies as described in Fig. 3.
Figure 4
Folding, oligomerization, and calnexin/calreticulin binding of single glycan deletion mutants. (A) Wild-type and single glycosylation mutants of HA were translated under oxidizing conditions in the presence of canine pancreas microsomes at 32°C for 1 h. At the indicated times, samples were removed, alkylated, lysed, and immunoprecipitated with anti-HA antibodies. HA was resolved by nonreducing SDS-PAGE and autoradiography. (B) Translations were performed as above with oxidation carried out for 8 h at 32°C. The alkylated HA was immunoprecipitated with anti-trimer specific HA antibodies and resolved by reducing SDS-PAGE and autoradiography. (C) Translation of 35S-labeled HA were performed as in A for 1 h at 32°C. After alkylation, HA was immunoprecipitated with anti-HA, -calnexin, and -calreticulin antibodies. HA was resolved upon nonreducing and reducing SDS-PAGE and autoradiography. The fraction HA coprecipitating with calnexin or calreticulin was calculated as the amount of anti-calnexin or -calreticulin precipitable HA divided by the total HA precipitated with anti-HA antibodies as described in Fig. 3.
Figure 6
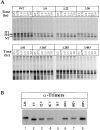
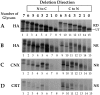
Determination of the number of glycans required for calnexin or calreticulin binding. Consensus glycosylation sequences were deleted by increasing, in single increments, from the NH2 to the COOH terminus (lanes 2–8), and from the COOH to the NH2 terminus (lanes 9–15). 35S-HA was translated in vitro for 1 h at 32°C, alkylated, lysed, and precipitated with anti-HA, -calnexin (CNX), or -calreticulin (CRT) antisera. The labeled HA was resolved by nonreducing (A, NR) and reducing (B–D, RD) SDS-PAGE and autoradiography. The band designated as UT corresponds to the untranslocated and unglycosylated HA.
Figure 5
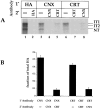
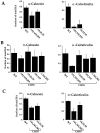
Mapping of calnexin and calreticulin binding sites by mutating consensus glycosylation sequences. The consensus sequences for glycosylation were eliminated by changing the Asn to Gln. The HA was translated under oxidizing conditions for 1 h at 32°C. (A) The fraction of total HA coprecipitating with the various antisera is plotted. (B) Binding to calnexin and calreticulin was monitored as described above for a set of HA mutants which had, in addition to mutations in the designated consensus glycosylation sites, an additional mutation, C305S, which arrested HA oxidation in the IT1 conformation. The disruption of disulfide 281– 305 by changing Cys305 to a Ser results in the accumulation of nonaggregated IT1 (not shown). The values given are the average of four experiments. (C) HA binding to calnexin and calreticulin in CHO cells was quantified as in A and B. 35S-HA was expressed in CHO cells by use of the T7-based vaccinia expression system. 4 h after infection, cells were pulsed with [35S]methionine/cysteine for 2 min at 37°C. Alkylated and lysed samples were then processed as above.
Figure 7
Cotranslational folding of wild-type and mutant HA, Δ8,22,38, and Δ81. CHO cells were infected with recombinant vaccinia virus and then transfected with the various HA genes. 4 h after transfection, the cells were starved in cysteine-/methionine-free medium for 30 min and then pulse labeled with [35S]cysteine/ methionine for 2 min. Immediately after labeling, the cells were lysed and precipitated with antibodies raised against a peptide corresponding to the NH2 terminus of HA. The precipitates were subjected to 2-D SDS-PAGE as in Fig. 2.
Figure 8
Binding of anchor− HA to calnexin and calreticulin. A soluble anchor-free form of HA was expressed truncated at the first transmembrane residue by replacing the codon for T514 with the stop codon, TGA. Wild-type and truncated (Anchor −) forms of HA were translated for 1 h at 32°C under oxidizing conditions in the presence of microsomes. After 1h, HA was alkylated, lysed, and precipitated with anti-HA, -calreticulin, and -calnexin antisera. HA was resolved, visualized, and quantified as in Fig. 3. Note the levels of chaperone binding in this experiment were higher throughout, likely due to the use of different antibody bleeds. This experiment was repeated three times with similar levels observed.
Similar articles
- Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins.
Peterson JR, Ora A, Van PN, Helenius A. Peterson JR, et al. Mol Biol Cell. 1995 Sep;6(9):1173-84. doi: 10.1091/mbc.6.9.1173. Mol Biol Cell. 1995. PMID: 8534914 Free PMC article. - Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes.
Hebert DN, Foellmer B, Helenius A. Hebert DN, et al. EMBO J. 1996 Jun 17;15(12):2961-8. EMBO J. 1996. PMID: 8670797 Free PMC article. - Chaperone selection during glycoprotein translocation into the endoplasmic reticulum.
Molinari M, Helenius A. Molinari M, et al. Science. 2000 Apr 14;288(5464):331-3. doi: 10.1126/science.288.5464.331. Science. 2000. PMID: 10764645 - Calnexin, calreticulin, and Bip/Kar2p in protein folding.
Hebert DN, Simons JF, Peterson JR, Helenius A. Hebert DN, et al. Cold Spring Harb Symp Quant Biol. 1995;60:405-15. doi: 10.1101/sqb.1995.060.01.045. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824414 Review. No abstract available. - Protein glucosylation and its role in protein folding.
Parodi AJ. Parodi AJ. Annu Rev Biochem. 2000;69:69-93. doi: 10.1146/annurev.biochem.69.1.69. Annu Rev Biochem. 2000. PMID: 10966453 Review.
Cited by
- ER chaperones use a protein folding and quality control glyco-code.
Guay KP, Ke H, Canniff NP, George GT, Eyles SJ, Mariappan M, Contessa JN, Gershenson A, Gierasch LM, Hebert DN. Guay KP, et al. Mol Cell. 2023 Dec 21;83(24):4524-4537.e5. doi: 10.1016/j.molcel.2023.11.006. Epub 2023 Dec 4. Mol Cell. 2023. PMID: 38052210 - Competition for calnexin binding regulates secretion and turnover of misfolded GPI-anchored proteins.
Cheatham AM, Sharma NR, Satpute-Krishnan P. Cheatham AM, et al. J Cell Biol. 2023 Oct 2;222(10):e202108160. doi: 10.1083/jcb.202108160. Epub 2023 Sep 13. J Cell Biol. 2023. PMID: 37702712 Free PMC article. - Accelerating Bayesian inference of dependency between mixed-type biological traits.
Zhang Z, Nishimura A, Trovão NS, Cherry JL, Holbrook AJ, Ji X, Lemey P, Suchard MA. Zhang Z, et al. PLoS Comput Biol. 2023 Aug 28;19(8):e1011419. doi: 10.1371/journal.pcbi.1011419. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37639445 Free PMC article. - Using the Sleeping Beauty (SB) Transposon to Generate Stable Cells Producing Enveloped Virus-Like Particles (eVLPs) Pseudotyped with SARS-CoV-2 Proteins for Vaccination.
Pszenny V, Tjhin E, Alves-Ferreira EVC, Spada S, Bouamr F, Nair V, Ganesan S, Grigg ME. Pszenny V, et al. Curr Protoc. 2022 Oct;2(10):e575. doi: 10.1002/cpz1.575. Curr Protoc. 2022. PMID: 36300895 Free PMC article. - N-Glycosylation on Asn50 of SND1 Is Required for Glioma U87 Cell Proliferation and Metastasis.
Zhou Y, Li Q, Zheng J, Lin N. Zhou Y, et al. J Immunol Res. 2022 Sep 29;2022:5239006. doi: 10.1155/2022/5239006. eCollection 2022. J Immunol Res. 2022. PMID: 36213325 Free PMC article.
References
- Arunachalam B, Cresswell P. Molecular requirements for the interaction of class II major histocompatibility complex molecules and invariant chain with calnexin. J Biol Chem. 1995;270:2784–2790. - PubMed
- Braakman I, Helenius J, Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature (Lond) 1992b;356:260–262. - PubMed
- Cannon KS, Hebert DN, Helenius A. Glycan-dependent and -independent association of vesicular stomatitis virus G protein with calnexin. J Biol Chem. 1996;271:14280–14284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials