Preferential termination of corticorubral axons on spine-like dendritic protrusions in developing cat - PubMed (original) (raw)
Preferential termination of corticorubral axons on spine-like dendritic protrusions in developing cat
Y Saito et al. J Neurosci. 1997.
Abstract
The formation of synaptic contacts is a crucial event during neural development and is thought to be achieved by complex interactions between incoming axons and the neurons in the target. We have focused on spine-like dendritic protrusions (SLDPs), which are transient pleomorphic protrusive structures seen in developing brains. Although the functional significance of SLDPs remains unknown, accumulating in vitro evidence suggests that the SLDP plays an important role in synaptogenetic interactions with axons. As a test of this idea, the present study was performed to examine whether the SLDPs are the preferential sites of synapse formation in vivo. The ultrastructure of biocytin-labeled corticorubral (CR) terminals was examined in serial thin sections during the period of synaptogenesis in newborn cats. We found that a major proportion (86%) of the CR synapses was formed on SLDPs. The presynaptic terminals were often invaginated by fine processes extending from the tips of SLDPs. Synaptic structures presumably of cortical origin were also found on SLDPs of HRP-labeled rubrospinal cells, suggesting that SLDPs postsynaptic to labeled CR terminals originate at least in part from rubrospinal cells. Taken together, these results indicate that SLDPs may represent preferred sites of synapse formation and support the notion that SLDPs play a role in synaptogenic interactions during brain development.
Figures
Fig. 1.
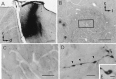
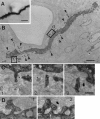
Identification of corticorubral fibers.A, Injection site of biocytin. A sagittal section of the sensorimotor cortex is shown. The arrow indicates the cruciate sulcus. r, Rostral; d, dorsal.B, Low-magnification photomicrograph of a horizontal section of the RN. Asterisks indicate the oculomotor nerve. c, Caudal; l, lateral.C, High-magnification photomicrograph of the area of the RN outlined by the rectangle in B. Many biocytin-labeled fibers can be seen. D,E, Higher-magnification photomicrographs of biocytin-labeled fibers. Axonal swellings are seen along the fibers (arrowheads). Some of the axonal swellings were fenestrated, as shown in E. Scale bars:A, 500 μm; B, 200 μm;C, 50 μm; D, 4 μm; E, 2 μm.
Fig. 8.
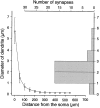
CR synaptic sites on the soma–dendritic membrane of RS cells. The graph to the left shows the diameter of HRP-labeled RS cell dendrites (n = 17) plotted against the distance from the soma. The_histogram_ on the right shows the distribution of the diameter of the dendrites from which synapse-bearing SLDPs emanated (n = 41). Comparison of the two graphs demonstrates that most of the synapses were located on dendritic regions <100 μm away from the soma.
Fig. 2.
Biocytin-labeled CR synapse on an SLDP. A, B, Electron micrographs from semiserial sections of a CR fiber. The CR fiber formed synapses (black arrow) on an SLDP emanating from a dendritic shaft (asterisk). SLDPs often contained vesicular structures (arrowheads) and smooth endoplasmic reticulum (small arrows). C, Low-magnification photomicrograph showing a filiform process emerging from a dendritic shaft (asterisk). Scale bars:A, B, 0.5 μm; C, 1 μm.
Fig. 3.
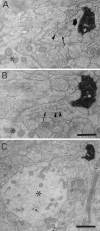
Biocytin-labeled CR axon terminal invaginated by an SLDP. A–D, Selected serial electron micrographs of a CR fiber. A, Low-magnification electron micrograph of a synapse-bearing dendritic protrusion. B–D, High-magnification electron micrographs of serial sections showing an SLDP encapsulated by a CR synaptic ending. The asterisk_shows the dendritic shaft. Arrows point to a synapse. Note that many vesicles can be seen within the SLDP (arrowheads). A single CR terminal rarely contacted both the SLDP and the dendrite. E, Three-dimensional reconstruction of CR axon terminal. Profiles shown in_tan and green represent the dendrite and the axon terminal, respectively. Other protrusions except for the one shown here were omitted for clarity. The protrusion has multiple branches and invaginates into the synaptic terminal. The_left_ and the center panels show the side view, and the right panel is the top view of the invaginated CR terminal. Scale bars: A, 1 μm;B–D, 0.5 μm.
Fig. 4.
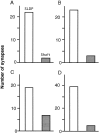
Localization of CR synapses on SLDPs.White and shaded bars represent the number of synapses on SLDPs and dendritic shafts, respectively.A–C, Data from individual kittens. A 50-μm-thick Epon block was cut into 5-μm-thick sections, and the areas with abundant axonal swellings were selected for thin sectioning. The lengths of the CR fibers analyzed in A–C are 1.1, 1.4, and 2.3 mm, respectively. D, Graph from a 50-μm-thick block directly cut into serial thin sections. In each case most of the synapses are found on SLDPs.
Fig. 5.
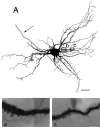
SLDPs emanating from a dendrite of an HRP-labeled RS cell. A, Drawing of an RS cell in a newborn kitten. The cell was reconstructed from 50-μm-thick horizontal sections. The_arrow_ shows the axon. B,C, Photomicrographs of the dendritic areas indicated by the two squares and letters in_A_. The photomicrographs in B and_C_ correspond to the areas outlined by _b_and c, respectively. Note that numerous protrusions extend from the dendritic shaft. Scale bars: A, 50 μm;B, C, 5 μm.
Fig. 6.
Synapses on SLDPs extending from an HRP-labeled RS cell dendrite. A, Photomicrograph of an RS cell dendrite. B, Low-magnification electron micrograph of the dendrite shown in A. Arrowheads point to labeled SLDPs. C, D, SLDPs emanating from the dendritic shaft form synapses (arrows) with unidentified endings. High-magnification electron micrographs of selected serial sections from the two areas outlined by_rectangles_ and letters in_B_. C and D correspond to the areas labeled by c and d in_B_, respectively. Granular profiles in HRP-stained dendrites are reaction products caused by the intensification procedure (see Materials and Methods). Scale bars: A, 5 μm;B, 2 μm; C, 0.5 μm; D, 0.25 μm.
Fig. 7.
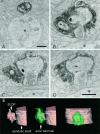
SLDPs of an HRP-labeled RS cell invaginating into an axon terminal. Asterisks show the dendritic shaft from which SLDPs emanate. The black arrow shows the synaptic terminal. A–H, Electron micrographs of selected serial sections. I, Three-dimensional reconstruction of the presynaptic axon terminal and the SLDP. See legend of Figure 3 for detail. Scale bar, 0.25 μm.
Fig. 9.
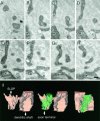
Synapses on the somatic membrane of RS cells.A, Electron micrograph of an HRP-filled RS cell.B, Higher magnification of the area outlined by the_rectangle_ in A. Note the presence of inclusions of synaptic endings in the soma. C, Synapses formed by terminals included in the soma (arrows). Similar inclusions were observed in nonstained soma, indicating that these are not artifacts of the HRP injection (not shown). Scale bars:A, 5 μm; B, 1 μm; C, 0.5 μm.
Similar articles
- Developing corticorubral axons of the cat form synapses on filopodial dendritic protrusions.
Saito Y, Murakami F, Song WJ, Okawa K, Shimono K, Katsumaru H. Saito Y, et al. Neurosci Lett. 1992 Nov 23;147(1):81-4. doi: 10.1016/0304-3940(92)90779-7. Neurosci Lett. 1992. PMID: 1480328 - Light and electron microscopic study of corticorubral synapses in adult cat: evidence for extensive synaptic remodeling during postnatal development.
Saito Y, Katsumaru H, Wilson CJ, Murakami F. Saito Y, et al. J Comp Neurol. 2001 Nov 19;440(3):236-44. doi: 10.1002/cne.1382. J Comp Neurol. 2001. PMID: 11745620 - Dendritic and somatic appendages of identified rubrospinal neurons of the cat.
Wilson CJ, Murakami F, Katsumaru H, Tsukahara N. Wilson CJ, et al. Neuroscience. 1987 Jul;22(1):113-30. doi: 10.1016/0306-4522(87)90202-8. Neuroscience. 1987. PMID: 2819771 - Synapses formed by ectopic corticofugal axons: an electron microscopic study of crossed corticorubral projections in kittens.
Murakami F, Saito Y, Higashi S, Oikawa H. Murakami F, et al. Neurosci Lett. 1991 Sep 30;131(1):49-52. doi: 10.1016/0304-3940(91)90334-p. Neurosci Lett. 1991. PMID: 1724305
Cited by
- AMPA receptors regulate experience-dependent dendritic arbor growth in vivo.
Haas K, Li J, Cline HT. Haas K, et al. Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):12127-31. doi: 10.1073/pnas.0602670103. Epub 2006 Aug 1. Proc Natl Acad Sci U S A. 2006. PMID: 16882725 Free PMC article. - Dendritic spines and development: towards a unifying model of spinogenesis--a present day review of Cajal's histological slides and drawings.
García-López P, García-Marín V, Freire M. García-López P, et al. Neural Plast. 2010;2010:769207. doi: 10.1155/2010/769207. Epub 2011 Mar 13. Neural Plast. 2010. PMID: 21584262 Free PMC article. - Neurite growth patterns leading to functional synapses in an identified embryonic neuron.
Reese D, Drapeau P. Reese D, et al. J Neurosci. 1998 Aug 1;18(15):5652-62. doi: 10.1523/JNEUROSCI.18-15-05652.1998. J Neurosci. 1998. PMID: 9671656 Free PMC article. - Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia.
Portera-Cailliau C, Pan DT, Yuste R. Portera-Cailliau C, et al. J Neurosci. 2003 Aug 6;23(18):7129-42. doi: 10.1523/JNEUROSCI.23-18-07129.2003. J Neurosci. 2003. PMID: 12904473 Free PMC article. - On the Role of Glutamate in Presynaptic Development: Possible Contributions of Presynaptic NMDA Receptors.
Fedder KN, Sabo SL. Fedder KN, et al. Biomolecules. 2015 Dec 14;5(4):3448-66. doi: 10.3390/biom5043448. Biomolecules. 2015. PMID: 26694480 Free PMC article. Review.
References
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. - PubMed
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. I. Quantitative analysis. J Neurocytol. 1983;12:599–616. - PubMed
- Boothe RG, Greenough WT, Lund JS, Wrege K. A quantitative investigation of spine and dendrite development of neurons in visual cortex (area 17) of Macaca nemestrina monkeys. J Comp Neurol. 1979;186:473–490. - PubMed
- Caceres A, Steward O. Dendritic reorganization in the denervated dentate gyrus of the rat following entorhinal cortical lesions: a Golgi and electron microscopic analysis. J Comp Neurol. 1983;214:387–403.
- Cooper MW, Smith SJ. A real-time analysis of growth cone-target cell interactions during the formation of stable contacts between hippocampal neurons in culture. J Neurobiol. 1992;23:814–828. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous