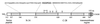Atomic structure of a thermostable subdomain of HIV-1 gp41 - PubMed (original) (raw)
Atomic structure of a thermostable subdomain of HIV-1 gp41
K Tan et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Infection by HIV-1 involves the fusion of viral and cellular membranes with subsequent transfer of viral genetic material into the cell. The HIV-1 envelope glycoprotein that mediates fusion consists of the surface subunit gp120 and the transmembrane subunit gp41. gp120 directs virion attachment to the cell-surface receptors, and gp41 then promotes viral-cell membrane fusion. A soluble, alpha-helical, trimeric complex within gp41 composed of N-terminal and C-terminal extraviral segments has been proposed to represent the core of the fusion-active conformation of the HIV-1 envelope. A thermostable subdomain denoted N34(L6)C28 can be formed by the N-34 and C-28 peptides connected by a flexible linker in place of the disulfide-bonded loop region. Three-dimensional structure of N34(L6)C28 reveals that three molecules fold into a six-stranded helical bundle. Three N-terminal helices within the bundle form a central, parallel, trimeric coiled coil, whereas three C-terminal helices pack in the reverse direction into three hydrophobic grooves on the surface of the N-terminal trimer. This thermostable subdomain displays the salient features of the core structure of the isolated gp41 subunit and thus provides a possible target for therapeutics designed selectively to block HIV-1 entry.
Figures
Figure 1
A thermostable, α-helical subdomain designated N34(L6)C28 within the extraviral portion of HIV-1 gp41. The single-chain recombinant N34(L6)C28 polypeptide consists of residues 546–579 (N-34) and 628–655 (C-28) of gp41 plus a linker of six hydrophilic residues (Ser-Gly-Gly-Arg-Gly-Gly). The important functional features of gp41 are shown. Expansion above the N-34 and C-28 peptides shows the amino acid sequence in single letter code. The disulfide bond and four potential N glycosylation sites are depicted. The residues are numbered according to their position in gp160.
Figure 2
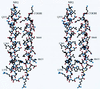
Structure of the N34(L6)C28 monomer. A stereo drawing of the N34(L6)C28 monomer. The carbon atoms are black, oxygen atoms are red, and nitrogen atoms are blue. The N and C termini of the molecule are labeled. The residues are shown in single letter code and numbered according to their position in gp160. Diagrams were prepared by using the program
molscript
(48).
Figure 3
Structure of the N34(L)C28 trimer. (A) Ribbon drawing of the trimer with the N terminus and C terminus of one molecule labeled. The N-terminal helices are colored yellow, whereas the C-terminal helices are purple. (B) An end-on view of N34(L6)C28 looking down the 3-fold axis of the trimer. The figure was generated with the program
molscript
(48).
Figure 4
Helix packing in the structure of the N34(L6)C28 trimer. (A) Packing in the interior, trimeric coiled coil. Successive a and d positions of the three N-terminal helices form nine layers of homotrimeric contacts. The side chains of the residues at the a and d positions are shown. The carbon atoms are black, oxygen atoms are red, and nitrogen atoms are blue. (B) Packing in the hydrophobic layer between Ala-558 and Ile-559 in the interior trimer and Leu-645 and Ile-646 in the outside C-terminal helices. Atoms are colored the same as in A. (C) Packing in the hydrophilic layer including Gln-550, Gln-551, Gln-552, and Asn-553 from the interior trimer, and Gln-652 and Gln-653 from the outside C-terminal helices. Residues are shown in single letter code. Only Q550, N553, Q652, and Q654 are labeled. Atoms are colored the same as in A. The figure was created with the program
molscript
(48).
Similar articles
- Subdomain folding and biological activity of the core structure from human immunodeficiency virus type 1 gp41: implications for viral membrane fusion.
Lu M, Ji H, Shen S. Lu M, et al. J Virol. 1999 May;73(5):4433-8. doi: 10.1128/JVI.73.5.4433-4438.1999. J Virol. 1999. PMID: 10196341 Free PMC article. - Core structure of gp41 from the HIV envelope glycoprotein.
Chan DC, Fass D, Berger JM, Kim PS. Chan DC, et al. Cell. 1997 Apr 18;89(2):263-73. doi: 10.1016/s0092-8674(00)80205-6. Cell. 1997. PMID: 9108481 - Helical interactions in the HIV-1 gp41 core reveal structural basis for the inhibitory activity of gp41 peptides.
Shu W, Liu J, Ji H, Radigen L, Jiang S, Lu M. Shu W, et al. Biochemistry. 2000 Feb 22;39(7):1634-42. doi: 10.1021/bi9921687. Biochemistry. 2000. PMID: 10677212 - Viral surface glycoproteins, gp120 and gp41, as potential drug targets against HIV-1: brief overview one quarter of a century past the approval of zidovudine, the first anti-retroviral drug.
Teixeira C, Gomes JR, Gomes P, Maurel F, Barbault F. Teixeira C, et al. Eur J Med Chem. 2011 Apr;46(4):979-92. doi: 10.1016/j.ejmech.2011.01.046. Epub 2011 Feb 3. Eur J Med Chem. 2011. PMID: 21345545 Review. - Peptide and non-peptide HIV fusion inhibitors.
Jiang S, Zhao Q, Debnath AK. Jiang S, et al. Curr Pharm Des. 2002;8(8):563-80. doi: 10.2174/1381612024607180. Curr Pharm Des. 2002. PMID: 11945159 Review.
Cited by
- Broad antiviral activity and crystal structure of HIV-1 fusion inhibitor sifuvirtide.
Yao X, Chong H, Zhang C, Waltersperger S, Wang M, Cui S, He Y. Yao X, et al. J Biol Chem. 2012 Feb 24;287(9):6788-96. doi: 10.1074/jbc.M111.317883. Epub 2012 Jan 6. J Biol Chem. 2012. PMID: 22228771 Free PMC article. - The M-T hook structure is critical for design of HIV-1 fusion inhibitors.
Chong H, Yao X, Sun J, Qiu Z, Zhang M, Waltersperger S, Wang M, Cui S, He Y. Chong H, et al. J Biol Chem. 2012 Oct 5;287(41):34558-68. doi: 10.1074/jbc.M112.390393. Epub 2012 Aug 9. J Biol Chem. 2012. PMID: 22879603 Free PMC article. - Discovery of critical residues for viral entry and inhibition through structural Insight of HIV-1 fusion inhibitor CP621-652.
Chong H, Yao X, Qiu Z, Qin B, Han R, Waltersperger S, Wang M, Cui S, He Y. Chong H, et al. J Biol Chem. 2012 Jun 8;287(24):20281-9. doi: 10.1074/jbc.M112.354126. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511760 Free PMC article. - Allosteric modulation of the HIV-1 gp120-gp41 association site by adjacent gp120 variable region 1 (V1) N-glycans linked to neutralization sensitivity.
Drummer HE, Hill MK, Maerz AL, Wood S, Ramsland PA, Mak J, Poumbourios P. Drummer HE, et al. PLoS Pathog. 2013;9(4):e1003218. doi: 10.1371/journal.ppat.1003218. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592978 Free PMC article. - Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1.
Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Yang X, et al. J Virol. 2005 Oct;79(19):12132-47. doi: 10.1128/JVI.79.19.12132-12147.2005. J Virol. 2005. PMID: 16160141 Free PMC article.
References
- Luciw P A. In: Fields Virology. Fields B N, Knipe D M, Howley P M, Chanock R M, Melinick J L, Monath T P, Roizman B, Straus S E, editors. Philadelphia: Lippincott; 1996. pp. 1881–1952.
- Nara P L, Garrity R R, Goudsmit J. FASEB J. 1991;5:2437–2455. - PubMed
- Freed E Q, Martin M A. J Biol Chem. 1995;270:23883–23886. - PubMed
- Wilkinson D. Curr Biol. 1996;6:1051–1053. - PubMed
- Wiley D C, Skehel J J. Annu Rev Biochem. 1987;56:365–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources