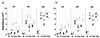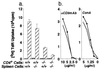T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice - PubMed (original) (raw)
T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice
H Wada et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Immunological functions were analyzed in mice lacking granulocyte/macrophage colony-stimulating factor (GM-CSF). The response of splenic T cells to allo-antigens, anti-mouse CD3 mAb, interleukin 2 (IL-2), or concanavalin A was comparable in GM-CSF +/+ and GM-CSF -/- mice. To investigate the responses of CD8(+) and CD4+ T cells against exogenous antigens, mice were immunized with ovalbumin peptide or with keyhole limpet hemocyanin (KLH). Cytotoxic CD8+ T cells with specificity for ovalbumin peptide could not be induced in GM-CSF -/- mice. After immunization with KLH, there was a delay in IgG generation, particularly IgG2a, in GM-CSF -/- mice. Purified CD4+ T cells from GM-CSF -/- mice immunized with KLH showed impaired proliferative responses and produced low amounts of interferon-gamma (IFN-gamma) and IL-4 when KLH-pulsed B cells or spleen cells were used as antigen presenting cells (APC). When enriched dendritic cells (DC) were used as APC, CD4+ T cells from GM-CSF -/- mice proliferated as well as those from GM-CSF +/+ mice and produced high amounts of IFN-gamma and IL-4. To analyze the rescue effect of DC on CD4(+) T cells, supernatants from (i) CD4(+) T cells cultured with KLH-pulsed DC or (ii) DC cultured with recombinant GM-CSF were transferred to cultures of CD4(+) T cells and KLH-pulsed spleen cells from GM-CSF -/- mice. Supernatants from both DC sources contained a factor or factors that restored proliferative responses and IFN-gamma production of CD4(+) T cells from GM-CSF -/- mice.
Figures
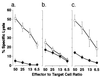
Figure 1
CTL and proliferative responses of naive GM-CSF +/+ and GM-CSF −/− spleen cells against BALB/c spleen cells. Spleen cells from GM-CSF +/+ (+/+) or GM-CSF −/− (−/−) mice were stimulated in vitro with mitomycin C-treated BALB/c spleen cells. (a) Cytotoxicity was assessed with 51Cr-labeled BALB/c RL♂1 (○) and C57BL EL4 (•) target cells. (b) Spleen cells from GM-CSF +/+ or GM-CSF −/− mice were stimulated in vitro with mitomycin C-treated autologous (▧) or BALB/c (▨) spleen cells. Proliferation was determined by incorporation of [_methyl_-3H]thymidine.
Figure 2
Generation of CTL against OVA peptide. Spleen cells of GM-CSF +/+ (a) or GM-CSF −/− mice (b and c) immunized with OVA peptide were stimulated with autologous spleen cells pulsed with OVA peptide and treated with mitomycin C. Cytotoxicity was assessed with 51Cr-labeled C57BL EL4 target cells pulsed with OVA peptide (○) or with no peptide (•). (c) The hind footpads of GM-CSF −/− mice were injected with 5 ng rGM-CSF and OVA peptide, followed by 10 ng/day rGM-CSF injected i.p. for 5 days, resulting in the generation of OVA-specific CTL .
Figure 3
Ab responses to KLH. (a) The hind footpads of GM-CSF −/− (•) and GM-CSF +/+ (○) mice were injected with 100 μg KLH in CFA. (b) The hind footpads of GM-CSF −/− mice were injected with 100 μg KLH with 40 ng rGM-CSF in CFA followed by 10 ng/day rGM-CSF injected i.p. for 5 days a week until mice were killed: GM-CSF −/− (•) and GM-CSF −/− with rGM-CSF (▵). Each group had four mice. Individual mice were bled at 1, 2, and 4 weeks after immunization and sera were titered for anti-KLH antibody at doubling dilutions of 1:500 to 1:16,000 using isotype-specific ELISA. The results of serum samples diluted 1:4,000 are plotted in this figure. Similar differences were seen with the other serum dilutions. Specificity of antibodies were confirmed using hen egg lysozyme as a negative control.
Figure 4
CD4+ T cell proliferative responses to KLH, immobilized anti-CD3 mAb, and Con A following immunization with KLH in CFA**.** (a) Purified CD4+ T cells from draining lymphnodes of immunized GM-CSF −/− or GM-CSF +/+ mice were stimulated with spleen cells pulsed with KLH (▨) or without KLH (▧) as indicated. Spleen cells were pulsed with 100 μg/ml KLH for 1 h. (b) Purified CD4+ T cells from immunized GM-CSF −/− (•) or GM-CSF +/+ (○) mice were stimulated with immobilized anti-CD3 mAb or Con A in vitro. Proliferation was determined by incorporation of [_methyl_-3H]thymidine.
Figure 5
DC and culture supernatants of DC stimulate the proliferative responses and IFN-γ production by CD4+ T cells from KLH-immunized GM-CSF −/− mice. (a) CD4+ T cells from KLH-immunized or naive mice were stimulated with autologous spleen cells or DC pulsed with KLH as indicated. (b) To analyze the effect of supernatants from DC, 100 μl supernatants were added to cultures of CD4+ T cells and KLH-pulsed spleen cells from immunized GM-CSF −/− mice at a final volume of 200 μl. Two sources of DC supernatants were used; (i) cocultures of immunized GM-CSF +/+ or GM-CSF −/− CD4+ T cells and KLH-pulsed autologous DC (mitomycin C-untreated) for 2 days, and (ii) cultures of DC from LPS-treated GM-CSF +/+ or GM-CSF −/− mice with 100 ng/ml rGM-CSF for 3 days. (iii) For control purposes, the effects of rGM-CSF or IL-12 added to cultures of CD4+ T cells and KLH-pulsed spleen cells from immunized GM-CSF −/− mice were also tested. Proliferation was determined by incorporation of [_methyl_-3H]thymidine and levels of IFN-γ in the culture supernatants were measured by ELISA.
Similar articles
- Regulation of IFN-gamma production in granulocyte-macrophage colony-stimulating factor-deficient mice.
Noguchi Y, Wada H, Marino MW, Old LJ. Noguchi Y, et al. Eur J Immunol. 1998 Dec;28(12):3980-8. doi: 10.1002/(SICI)1521-4141(199812)28:12<3980::AID-IMMU3980>3.0.CO;2-D. Eur J Immunol. 1998. PMID: 9862334 - Dendritic cells generated in the presence of granulocyte-macrophage colony-stimulating factor and IFN-alpha are potent inducers of HIV-specific CD8 T cells.
Carbonneil C, Aouba A, Burgard M, Cardinaud S, Rouzioux C, Langlade-Demoyen P, Weiss L. Carbonneil C, et al. AIDS. 2003 Aug 15;17(12):1731-40. doi: 10.1097/00002030-200308150-00002. AIDS. 2003. PMID: 12891059 - Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response.
Kwak LW, Young HA, Pennington RW, Weeks SD. Kwak LW, et al. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):10972-7. doi: 10.1073/pnas.93.20.10972. Proc Natl Acad Sci U S A. 1996. PMID: 8855293 Free PMC article. - Granulocyte-macrophage colony-stimulating factor as a mediator of autoimmunity in multiple sclerosis.
Kostic M, Zivkovic N, Cvetanovic A, Stojanovic I. Kostic M, et al. J Neuroimmunol. 2018 Oct 15;323:1-9. doi: 10.1016/j.jneuroim.2018.07.002. Epub 2018 Jul 5. J Neuroimmunol. 2018. PMID: 30196820 Review. - The sensitization phase of T-cell-mediated immunity.
Steinman RM, Koide S, Witmer M, Crowley M, Bhardwaj N, Freudenthal P, Young J, Inaba K. Steinman RM, et al. Ann N Y Acad Sci. 1988;546:80-90. doi: 10.1111/j.1749-6632.1988.tb21622.x. Ann N Y Acad Sci. 1988. PMID: 3073702 Review.
Cited by
- Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells.
Thepmalee C, Panya A, Sujjitjoon J, Sawasdee N, Poungvarin N, Junking M, Yenchitsomanus PT. Thepmalee C, et al. Hum Vaccin Immunother. 2020 Oct 2;16(10):2318-2327. doi: 10.1080/21645515.2019.1701913. Epub 2020 Jan 24. Hum Vaccin Immunother. 2020. PMID: 31976810 Free PMC article. - Disrupting Smad3 potentiates immunostimulatory function of NK cells against lung carcinoma by promoting GM-CSF production.
Lian GY, Wang QM, Mak TS, Huang XR, Yu XQ, Lan HY. Lian GY, et al. Cell Mol Life Sci. 2024 Jun 15;81(1):262. doi: 10.1007/s00018-024-05290-4. Cell Mol Life Sci. 2024. PMID: 38878186 Free PMC article. - Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis.
McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. McQualter JL, et al. J Exp Med. 2001 Oct 1;194(7):873-82. doi: 10.1084/jem.194.7.873. J Exp Med. 2001. PMID: 11581310 Free PMC article. - Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage.
Ushach I, Zlotnik A. Ushach I, et al. J Leukoc Biol. 2016 Sep;100(3):481-9. doi: 10.1189/jlb.3RU0316-144R. Epub 2016 Jun 28. J Leukoc Biol. 2016. PMID: 27354413 Free PMC article. Review. - GM-CSF: A Double-Edged Sword in Cancer Immunotherapy.
Kumar A, Taghi Khani A, Sanchez Ortiz A, Swaminathan S. Kumar A, et al. Front Immunol. 2022 Jul 5;13:901277. doi: 10.3389/fimmu.2022.901277. eCollection 2022. Front Immunol. 2022. PMID: 35865534 Free PMC article. Review.
References
- Moore M A S. Annu Rev Immunol. 1991;9:159–191. - PubMed
- Lieschke G J, Burgess A W. N Engl J Med. 1992;327:28–35. - PubMed
- Disis M L, Bernhard H, Shiota F M, Hand S L, Gralow J R, Huseby E S, Gillis S, Cheever M A. Blood. 1996;88:202–210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials