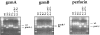In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A x B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells - PubMed (original) (raw)
In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A x B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells
M M Simon et al. J Exp Med. 1997.
Abstract
Granzyme (gzm) A and gzmB have been implicated in Fas-independent nucleolytic and cytolytic processes exerted by cytotoxic T (Tc) cells, but the underlying mechanism(s) remains unclear. In this study, we compare the potential of Tc and natural killer (NK) cells of mice deficient in both gzmA and B (gzmAxB-/-) with those from single knockout mice deficient in gzmA (-/-), gzmB (-/-), or perforin (-/-) to induce nuclear damage and lysis in target cells. With the exception of perforin-/-, all in vitro- and ex vivo-derived Tc and NK cell populations from the mutant strains induced 51Cr-release in target cells at levels and with kinetics similar to those of normal mice. This contrasts with their capacity to induce apoptotic nuclear damage in target cells. In gzmAxB-/- mice, Tc/NK-mediated target cell DNA fragmentation was not observed, even after extended incubation periods (10 h), but was normal in gzmA-deficient and only impaired in gzmB-deficient mice in short-term (2-4 h), but not long-term (4-10 h), nucleolytic assays. This suggests that gzmA and B are critical for Tc/NK granule- mediated nucleolysis, with gzmB being the main contributor, while target cell lysis is due solely to perforin and independent of both proteases.
Figures
Figure 1
Analysis of wild-type (C57BL/ 6) and the mutant mice gzmA−/−, gzmB−/−, gzmA×B−/−, and perforin−/− mice by PCR. Tail DNA from individual mice was analyzed by PCR amplification using the gzmA-, gzmB-, and perforin-specific primer pairs, as indicated in Materials and Methods.
Figure 2
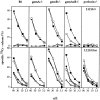
51Cr-release (top) and 125I-DNA release (bottom) of L1210 (A and E), L1210.Fas (B), A1.1 (C), and TA3 (D) target cells induced by in vitro– derived alloreactive Tc cells. Splenocytes from B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice (pools of two spleens per mouse strain, A–D; (E) spleens of four individual gzmA×B−/− (▪), one B6 mouse (•), and one perforin−/− mouse (*) were activated in primary MLC (A–D, E, top) or secondary MLC (E, bottom), and tested for cytolytic (top) and nucleolytic (bottom) activities for the indicated time periods. All values are the mean lysis of triplicate samples at three e/t values given. SEM never exceeded 3%.
Figure 2
51Cr-release (top) and 125I-DNA release (bottom) of L1210 (A and E), L1210.Fas (B), A1.1 (C), and TA3 (D) target cells induced by in vitro– derived alloreactive Tc cells. Splenocytes from B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice (pools of two spleens per mouse strain, A–D; (E) spleens of four individual gzmA×B−/− (▪), one B6 mouse (•), and one perforin−/− mouse (*) were activated in primary MLC (A–D, E, top) or secondary MLC (E, bottom), and tested for cytolytic (top) and nucleolytic (bottom) activities for the indicated time periods. All values are the mean lysis of triplicate samples at three e/t values given. SEM never exceeded 3%.
Figure 3
51Cr-release from L1210.3 (top) and L1210.Fas (bottom) target cells induced by in vivo–derived alloreactive splenic effector cells from two individual (open and closed symbols) mutant or B6 mice in the absence (squares) and presence (circles) of EGTA. Mice were immunized intraperitoneally with 107 PFU of vaccinia virus encoding Kd 6 d before removal of spleen. All assays were harvested after 6 h. All values are the mean lysis of triplicate samples at the four e/t values given. SEM never exceeded 3%.
Figure 4
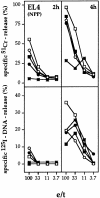
51Cr-release (top) and 125I-DNA release (bottom) from NPP-modified EL4 target cells by ex vivo–derived influenza virus A/WSN–immune Tc from mutant or B6 mice. B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice were infected intravenously with influenza virus A/WSN and their splenocytes (pool of three mice) were tested after 6 d for cytolytic (top) and nucleolytic (bottom) activities on NPP-modified EL4 target cells. Assay times were 2 and 4 h. All values are the mean lysis of triplicate samples at the four e/t values given. SEM never exceeded 3%.
Figure 5
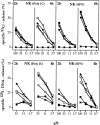
51Cr-release (top) and 125I-DNA release (bottom) from YAC-1 target cells by ex vivo–derived NK cells from mutant or B6 mice. B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice were either treated with poly I:C (20 h) or infected with SFV (2 d) and their splenocytes (poly I:C, pool of two spleens; SFV, pool of three spleens) were tested for cytolytic (top) or nucleolytic (bottom) activities on YAC-1 target cells. Assay times were 2 and 4 h. All values are the mean lysis of triplicate samples at three or four e/t values given. SEM never exceeded 3%.
Similar articles
- Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis.
Pardo J, Bosque A, Brehm R, Wallich R, Naval J, Müllbacher A, Anel A, Simon MM. Pardo J, et al. J Cell Biol. 2004 Nov 8;167(3):457-68. doi: 10.1083/jcb.200406115. J Cell Biol. 2004. PMID: 15534000 Free PMC article. - Cytotoxic T cells specifically induce Fas on target cells, thereby facilitating exocytosis-independent induction of apoptosis.
Simon MM, Waring P, Lobigs M, Nil A, Tran T, Hla RT, Chin S, Müllbacher A. Simon MM, et al. J Immunol. 2000 Oct 1;165(7):3663-72. doi: 10.4049/jimmunol.165.7.3663. J Immunol. 2000. PMID: 11034370 - Granzymes are essential for natural killer cell-mediated and perf-facilitated tumor control.
Pardo J, Balkow S, Anel A, Simon MM. Pardo J, et al. Eur J Immunol. 2002 Oct;32(10):2881-7. doi: 10.1002/1521-4141(2002010)32:10<2881::AID-IMMU2881>3.0.CO;2-K. Eur J Immunol. 2002. PMID: 12355441 - Killing by cytotoxic T cells and natural killer cells: multiple granule serine proteases as initiators of DNA fragmentation.
Trapani JA, Smyth MJ. Trapani JA, et al. Immunol Cell Biol. 1993 Jun;71 ( Pt 3):201-8. doi: 10.1038/icb.1993.22. Immunol Cell Biol. 1993. PMID: 8349302 Review. - Cell-mediated cytotoxicity in recovery from poxvirus infections.
Müllbacher A. Müllbacher A. Rev Med Virol. 2003 Jul-Aug;13(4):223-32. doi: 10.1002/rmv.381. Rev Med Virol. 2003. PMID: 12820184 Review.
Cited by
- Hemizygous Granzyme A Mice Expressing the hSOD1G93A Transgene Show Slightly Extended Lifespan.
Moreno-Martinez L, Santiago L, de la Torre M, Calvo AC, Pardo J, Osta R. Moreno-Martinez L, et al. Int J Mol Sci. 2022 Nov 4;23(21):13554. doi: 10.3390/ijms232113554. Int J Mol Sci. 2022. PMID: 36362341 Free PMC article. - IL-15 and PIM kinases direct the metabolic programming of intestinal intraepithelial lymphocytes.
James OJ, Vandereyken M, Marchingo JM, Singh F, Bray SE, Wilson J, Love AG, Swamy M. James OJ, et al. Nat Commun. 2021 Jul 13;12(1):4290. doi: 10.1038/s41467-021-24473-2. Nat Commun. 2021. PMID: 34257288 Free PMC article. - Studying the biology of cytotoxic T lymphocytes in vivo with a fluorescent granzyme B-mTFP knock-in mouse.
Chitirala P, Chang HF, Martzloff P, Harenberg C, Ravichandran K, Abdulreda MH, Berggren PO, Krause E, Schirra C, Leinders-Zufall T, Benseler F, Brose N, Rettig J. Chitirala P, et al. Elife. 2020 Jul 22;9:e58065. doi: 10.7554/eLife.58065. Elife. 2020. PMID: 32696761 Free PMC article. - T Cells and Regulated Cell Death: Kill or Be Killed.
Spetz J, Presser AG, Sarosiek KA. Spetz J, et al. Int Rev Cell Mol Biol. 2019;342:27-71. doi: 10.1016/bs.ircmb.2018.07.004. Epub 2018 Aug 29. Int Rev Cell Mol Biol. 2019. PMID: 30635093 Free PMC article. Review. - When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection.
Belizário JE, Neyra JM, Setúbal Destro Rodrigues MF. Belizário JE, et al. Innate Immun. 2018 Nov;24(8):452-465. doi: 10.1177/1753425918800200. Epub 2018 Sep 20. Innate Immun. 2018. PMID: 30236030 Free PMC article. Review.
References
- Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature (Lond) 1994;369:31–37. - PubMed
- Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell–mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. - PubMed
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous