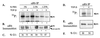Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb - PubMed (original) (raw)
Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb
S A Ezhevsky et al. Proc Natl Acad Sci U S A. 1997.
Abstract
In cycling cells, the retinoblastoma protein (pRb) is un- and/or hypo-phosphorylated in early G1 and becomes hyper-phosphorylated in late G1. The role of hypo-phosphorylation and identity of the relevant kinase(s) remains unknown. We show here that hypo-phosphorylated pRb associates with E2F in vivo and is therefore active. Increasing the intracellular concentration of the Cdk4/6 specific inhibitor p15(INK4b) by transforming growth factor beta treatment of keratinocytes results in G1 arrest and loss of hypo-phosphorylated pRb with an increase in unphosphorylated pRb. Conversely, p15(INK4b)-independent transforming growth factor beta-mediated G1 arrest of hepatocellular carcinoma cells results in loss of Cdk2 kinase activity with continued Cdk6 kinase activity and pRb remains only hypo-phosphorylated. Introduction of the Cdk4/6 inhibitor p16(INK4a) protein into cells by fusion to a protein transduction domain also prevents pRb hypo-phosphorylation with an increase in unphosphorylated pRb. We conclude that cyclin D:Cdk4/6 complexes hypo-phosphorylate pRb in early G1 allowing continued E2F binding.
Figures
Figure 1
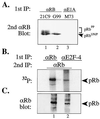
E2F-4 interacts with hypo-phosphorylated pRb in vivo. (A) Antibody controls of immunoprecipitated and immunoblotted pRb from asynchronously cycling HaCat cells using 21C9 anti-pRb antibodies that recognize all forms of pRb (lane 1), conformational specific G99 anti-pRb antibodies that recognize only the fastest migrating, un- and hypo-phosphorylated pRb forms (lane 2), and nonspecific control M73 anti-E1A antibodies (lane 3). Please note the complete absence of immunoprecipitated pRb in lane 3 (HaCaT cells do not express E1A). (B and C) HaCaT human keratinocytes (1 × 108) were labeled with 100 mCi [32P]orthophosphate and primary immunoprecipitation was performed with either anti-E2F-4 (90% of total lysate) or with G99 anti-pRb (10% of total lysate) antibodies followed by secondary immunoprecipitation with rabbit anti-pRb antibodies, resolved by SDS/PAGE, transferred to a filter, and analyzed for 32P content (B) and then probed with anti-pRb antibodies to normalize for pRb protein levels (C).
Figure 2
TGF-β treatment of cycling and contact inhibited G1-arrested HaCaT cells results in a reduction in the amount of in vivo hypo-phosphorylation of pRb. (A_–_C) Low-density asynchronous cycling and (D–F) high density contact-inhibited G1-arrested HaCaT cells were treated with TGF-β for 36 hr, labeled with [32P]orthophosphate, and immunoprecipitated with G99 anti-pRb antibodies that recognize the fastest migrating forms of pRb. Immune complexes were resolved by SDS/PAGE, transferred to a filter, and analyzed for 32P content (A and D) and then the same filter was probed with anti-pRb antibodies (B and E) to normalize for pRb protein levels as indicated. (C and F) Percentage of cells in G1 determined by FACS analysis.
Figure 3
TGF-β treatment of HaCaT cells results in the loss of a single tryptic phosphopeptide species detected on the remaining low level of hypo-phosphorylated pRb species. Two-dimensional tryptic phosphopeptide mapping of in vivo [32P]orthophosphate-labeled pRb from G99 anti-pRb immune complexes from low density control (A) and TGF-β-treated (B) cultures of HaCaT cells. Arrow indicates the loss of a phosphorylated pRb tryptic peptide. TGF-β and control samples were loaded based on approximately equal cpm of 32P and not moles of pRb
Figure 4
Transduction of full-length p16 protein directly into cells results in loss of pRb hypo-phosphorylation. (A) Comparison of purity and concentration of bacterially expressed wild-type and mutant TAT-p16 purified fusion proteins by Coomassie blue staining. (B) High-density 36-hr contact-inhibited HaCaT cells were replated at low density and treated with either wild-type or mutant TAT-p16 protein at a final concentration of 300 nM. Cells were analyzed for cell cycle position by FACS at 30-hr postreplating. (C) p16INK4a(−) Jurkat T cells were transduced with either wild-type or mutant TAT-p16 protein, labeled with [35S]methionine, and immunoprecipitated with anti-p16 antibodies, then re-immunoprecipitated with anti-Cdk6 antibodies and resolved by SDS/PAGE. The position of Cdk6 is indicated. (D and E) Wild-type and mutant TAT-p16 proteins were added to high density 36-hr contact-inhibited HaCaT cells for 1 hr and then [32P]orthophosphate labeled for 5 hr in the presence of TAT-p16. Cellular lysates were prepared and pRb was immunoprecipitated with G99 anti-pRb antibodies, transferred to a filter, exposed to a PhosphorImager screen for 32P content (D), and then the same filter was probed with anti-pRb antibodies (E) to normalize for pRb protein levels.
Figure 5
HepG2 hepatocellular carcinoma cells that arrest in G1 with TGF-β treatment in a p15INK4b-independent manner do not alter the in vivo hypo-phosphorylation of pRb. (A) DNA content by FACS analysis of asynchronous HepG2 cells treated with TGF-β for 48 hr. (B) Anti-pRb immunoblot analysis of control and TGF-β treated HepG2 cellular lysates with anti-pRb antibodies at 24 and 48 hr. (C) Anti-cdk2 and (D) anti-cdk6 and control rabbit anti-mouse IgG (RαM) immunoprecipitation-kinase assay from control and TGF-β treated HepG2 lysates using histone H1 and GST–RB–C′ as in vitro substrates, respectively. (E and F) Asynchronous HepG2 cells were treated with TGF-β for 48 hr, labeled in vivo with [32P]orthophosphate, and immunoprecipitated with G99 anti-pRb antibodies that recognize the fastest migrating forms of pRb. Immune complexes were resolved by SDS/PAGE, transferred to a filter, and analyzed for 32P content (E) and then the same filter was probed with anti-pRb antibodies (F) to normalize for pRb protein levels as indicated.
Figure 6
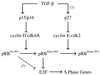
Model of p15/16, cyclin D:Cdk4/6, pRb, E2F pathway. Cyclin D:Cdk4/6 complexes associate with pRbs pocket domain and then proceed to hypo-phosphorylate pRb in early G1, and likely throughout the entire cell cycle. Hypo-phosphorylated pRb is active and binds to transcription factors, such as E2Fs. The initial hyper-phosphorylating inactivator of pRb is likely cyclin E:Cdk2 complexes expressed and activated at a position congruent with the passage through the late G1 restriction point. Hyper-phosphorylation of pRb results in the dissociation of E2Fs and subsequent activation of E2F-specific promoters, such as genes required for DNA synthesis. The presence of unphosphorylated pRb in TGF-β-treated wild-type cells and the apparent requirement of cyclin E:cdk complexes for prior hypo-phosphorylated pRb as an in vivo substrate (37) suggests that TGF-β signaling drives the cell further into a G0-like state.
References
- Weinberg R A. Cell. 1995;81:323–330. - PubMed
- Sherr C J. Science. 1996;274:1672–1677. - PubMed
- Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. - PubMed
- Bagchi S, Weinmann R, Raychaudhuri P. Cell. 1991;65:1063–1072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources