Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene - PubMed (original) (raw)
Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene
M Yamada et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Thyrotropin-releasing hormone (TRH) is a brain hypothalamic hormone that regulates thyrotropin (TSH) secretion from the anterior pituitary and is ubiquitously distributed throughout the brain and other tissues including pancreas. To facilitate studies into the role of endogenous TRH, we have used homologous recombination to generate mice that lack TRH. These TRH-/- mice are viable, fertile, and exhibit normal development. However, they showed obvious hypothyroidism with characteristic elevation of serum TSH level and diminished TSH biological activity. Their anterior pituitaries exhibited an apparent decrease in TSH immunopositive cells that was not due to hypothyroidism. Furthermore, this decrease could be reversed by TRH, but not thyroid hormone replacement, suggesting a direct involvement of TRH in the regulation of thyrotrophs. The TRH-/- mice also exhibited hyperglycemia, which was accompanied by impaired insulin secretion in response to glucose. These findings indicate that TRH-/- mice provide a model of exploiting tertiary hypothyroidism, and that TRH gene abnormalities cause disturbance of insulin secretion resulting in marked hyperglycemia.
Figures
Figure 1
Targeted disruption of the pTRH gene. (a) Schematic representation of the mouse pTRH gene (Upper), targeting vector, expected targeted allele, and pTRH (Lower). Solid boxes represent exons. Allele-specific PCR primers for selection of homologous recombinants are indicated by arrows, and probes for Southern and Northern blot analyses are also indicated. Hatched bars represent TRH progenitor sequences in pTRH. (b) Southern blot analysis of DNA from ES cell clones and siblings generated by crossing heterozygous mice (F2 mice). The wild-type (3.1 kb) and targeted (2.0 kb) _Hin_dIII and _Eco_RI fragments were detected by the 3′ external probe indicated in a. (c) Analysis of pTRH mRNA expression by Northern blot analysis of F2 mice. Total RNAs (20 μg) from the hypothalamus of wild-type (+/+), heterozygous (+/−), and homozygous mice (−/−) were hybridized with pTRH cRNA. (d) Hypothalamic TRH contents of the wild-type (+/+, n = 9), heterozygous (+/−, n = 5), and homozygous (−/−, n = 10) mice. ∗∗∗, P < 0.001 vs. wild type.
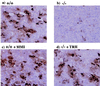
Figure 2
Immunohistochemical analysis of the pituitary. When compared with those of the wild type (a), numbers of TSH positive cells in the TRH−/− pituitary (b) were decreased. This decrease was restored by TRH replacement (d). No significant change was found in the pituitary of the hypothyroid mice induced by MMI treatment (c).
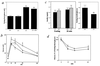
Figure 3
TRH stimulation test. After TRH administration, blood TSH (a) and T3 levels (b) were determined in the wild-type (+/+, n = 6) and homozygous (−/−, n = 6) mice. The increment in T3 over the basal level was reduced in the homozygotes.
Figure 4
Blood glucose and plasma insulin levels in TRH−/− mice. (a) Fasting blood glucose levels in the wild-type (+/+, n = 11), heterozygous (+/−, n = 10), homozygous (−/−, n = 10) mice, and homozygous mice with T4 replacement (−/− + T4, n = 7). ∗∗, P < 0.01 vs. the wild-type and heterozygous mice. (b) Glucose tolerance tests. Blood glucose levels were determined after glucose challenge. Data indicate means ± SEM (n = 10–15). (▵), wild type; (▪), heterozygotes; (○), homozygotes. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. wild type. (c) Plasma insulin levels before and 30 min after glucose challenge, and the ratio of fasting plasma insulin to glucose. +/+, wild type, n = 7; −/−, homozygotes, n = 7. ∗∗, P< 0.01 vs. wild type. (d) Insulin tolerance tests. Results are presented as the percentage of the fasting blood glucose and are means ± SEM. (○), wild type, n = 5; (▴), homozygotes, n = 5.
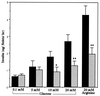
Figure 5
Characterization of TRH−/− islets. Insulin secretion in response to glucose and arginine was determined in vitro. Values are expressed as means ± SEM (n = 10–15). Solid bars, wild type; hatched bars, homozygotes. ∗, P < 0.05; ∗∗, P < 0.01 vs. wild type.
Similar articles
- Requirement of thyrotropin-releasing hormone for the postnatal functions of pituitary thyrotrophs: ontogeny study of congenital tertiary hypothyroidism in mice.
Shibusawa N, Yamada M, Hirato J, Monden T, Satoh T, Mori M. Shibusawa N, et al. Mol Endocrinol. 2000 Jan;14(1):137-46. doi: 10.1210/mend.14.1.0404. Mol Endocrinol. 2000. PMID: 10628753 - Mice lacking the thyrotropin-releasing hormone gene: what do they tell us?
Yamada M, Satoh T, Mori M. Yamada M, et al. Thyroid. 2003 Dec;13(12):1111-21. doi: 10.1089/10507250360731505. Thyroid. 2003. PMID: 14751031 Review. - Low setting of feedback regulation of TSH secretion by thyroxine in pituitary dwarfism with TSH-releasing hormone deficiency.
Sato T, Ishiguro K, Suzuki Y, Taketani T, Isumisawa A. Sato T, et al. J Clin Endocrinol Metab. 1976 Feb;42(2):385-90. doi: 10.1210/jcem-42-2-385. J Clin Endocrinol Metab. 1976. PMID: 816807 - Thyrotropin-releasing hormone stimulates growth hormone release from the anterior pituitary of hypothyroid rats in vitro.
Szabo M, Stachura ME, Paleologos N, Bybee DE, Frohman LA. Szabo M, et al. Endocrinology. 1984 Apr;114(4):1344-51. doi: 10.1210/endo-114-4-1344. Endocrinology. 1984. PMID: 6423373 - Thyrotropin-releasing hormone and thyrotropin secretion.
Utiger RD. Utiger RD. J Lab Clin Med. 1987 Mar;109(3):327-35. J Lab Clin Med. 1987. PMID: 3102657 Review.
Cited by
- In uncontrolled diabetes, thyroid hormone and sympathetic activators induce thermogenesis without increasing glucose uptake in brown adipose tissue.
Matsen ME, Thaler JP, Wisse BE, Guyenet SJ, Meek TH, Ogimoto K, Cubelo A, Fischer JD, Kaiyala KJ, Schwartz MW, Morton GJ. Matsen ME, et al. Am J Physiol Endocrinol Metab. 2013 Apr 1;304(7):E734-46. doi: 10.1152/ajpendo.00488.2012. Epub 2013 Feb 5. Am J Physiol Endocrinol Metab. 2013. PMID: 23384771 Free PMC article. - Glutaminyl cyclase knock-out mice exhibit slight hypothyroidism but no hypogonadism: implications for enzyme function and drug development.
Schilling S, Kohlmann S, Bäuscher C, Sedlmeier R, Koch B, Eichentopf R, Becker A, Cynis H, Hoffmann T, Berg S, Freyse EJ, von Hörsten S, Rossner S, Graubner S, Demuth HU. Schilling S, et al. J Biol Chem. 2011 Apr 22;286(16):14199-208. doi: 10.1074/jbc.M111.229385. Epub 2011 Feb 17. J Biol Chem. 2011. PMID: 21330373 Free PMC article. - The Thyrotropin-Releasing Hormone-Degrading Ectoenzyme, a Therapeutic Target?
Charli JL, Rodríguez-Rodríguez A, Hernández-Ortega K, Cote-Vélez A, Uribe RM, Jaimes-Hoy L, Joseph-Bravo P. Charli JL, et al. Front Pharmacol. 2020 May 8;11:640. doi: 10.3389/fphar.2020.00640. eCollection 2020. Front Pharmacol. 2020. PMID: 32457627 Free PMC article. Review. - The hypothalamic-pituitary-thyroid axis is intact in male insulin receptor substrate 4 knockout mice.
Brûlé E, Zhou X, Wang Y, Buddle ERS, Ongaro L, Loka M, Boelen A, Bernard D. Brûlé E, et al. Eur Thyroid J. 2024 Jan 1;13(1):e230054. doi: 10.1530/ETJ-23-0054. Online ahead of print. Eur Thyroid J. 2024. PMID: 38271814 Free PMC article. - Reversal of physiological deficits caused by diminished levels of peptidylglycine alpha-amidating monooxygenase by dietary copper.
Bousquet-Moore D, Ma XM, Nillni EA, Czyzyk TA, Pintar JE, Eipper BA, Mains RE. Bousquet-Moore D, et al. Endocrinology. 2009 Apr;150(4):1739-47. doi: 10.1210/en.2008-1202. Epub 2008 Nov 20. Endocrinology. 2009. PMID: 19022883 Free PMC article.
References
- Boler J, Enzmann F, Folkers K, Bowers C Y, Schally A V. Biochem Biophys Res Commun. 1969;37:705–710. - PubMed
- Burgus R, Dunn T F, Desiderio D, Guillemin R. C R Hebd Acad Sci Seances D. 1969;269:1870–1873. - PubMed
- Jackson I M. N Engl J Med. 1982;306:145–155. - PubMed
- Morley J E. Life Sci. 1979;25:1539–1550. - PubMed
- Metcalf G. Ann NY Acad Sci. 1989;553:311–313.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases