Heme oxygenase 1 is required for mammalian iron reutilization - PubMed (original) (raw)
Heme oxygenase 1 is required for mammalian iron reutilization
K D Poss et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The majority of iron for essential mammalian biological activities such as erythropoiesis is thought to be reutilized from cellular hemoproteins. Here, we generated mice lacking functional heme oxygenase 1 (Hmox1; EC 1.14.99.3), which catabolizes heme to biliverdin, carbon monoxide, and free iron, to assess its participation in iron homeostasis. Hmox1-deficient adult mice developed an anemia associated with abnormally low serum iron levels, yet accumulated hepatic and renal iron that contributed to macromolecular oxidative damage, tissue injury, and chronic inflammation. Our results indicate that Hmox1 has an important recycling role by facilitating the release of iron from hepatic and renal cells, and describe a mouse model of human iron metabolic disorders.
Figures
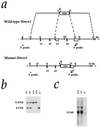
Figure 1
Targeted disruption of the Hmox1 gene. (a) Hmox1 genomic locus and targeting vector. A 3.7-kb region including exons 3, 4, and a portion of 5 (e3–e5) was replaced with a pgk-neo cassette. The 5′ and 3′ probes used for screening ES cell clones and genotyping mice are shown. The 5′ probe hybridizes to an 11-kb _Kpn_I fragment of the native Hmox1 gene and a 6.5-kb fragment from the disrupted gene. D, _Hin_dIII site; K, _Kpn_I; X, _Xho_I; Xb, _Xba_I. (b) Southern blot analysis of _Kpn_I-digested tail DNA from ES cell-derived mice. The blot was hybridized with the 5′ Hmox1 probe. Genotypes of one Hmox1 homozygous mutant mouse (−/−), two wild-type mice (+/+), and two heterozygous mice (+/−) are indicated. (c) Northern blot analysis of total splenic RNA from a wild-type mouse (+/+), a heterozygous mouse (+/−), and a homozygous mutant mouse (−/−). The blot was hybridized with a rat Hmox1 cDNA probe (13), which recognizes a major mRNA band of approximately 1.5 kb. The Hmox1 probe recognized an aberrantly sized mRNA from _Hmox1_−/− mice that was barely detectable even in an overloaded lane.
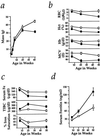
Figure 2
Wasting, anemia, and serum iron deficiency in _Hmox1_−/− mice. (a) _Hmox1_−/− mice were consistently slightly smaller than Hmox1+/− littermates, but weight loss between 20 and 40 weeks of age was clearly indicative of wasting. Data are shown mean ± SEM. One and 3 weeks, 9 _Hmox1_−/− and 19 Hmox1+/− males and females were analyzed; 6 weeks, 12 _Hmox1_−/− and 24 Hmox1+/− males only; 20 weeks, 9 _Hmox1_−/− and 11 _Hmox1_−/− males; and 40 weeks, 13 _Hmox1_−/− and 15 Hmox1+/− males. Significant differences (P < 0.05) were observed between _Hmox1_−/− and Hmox1+/− masses at all ages. In a–d, □, Hmox1+/− mean values; ▪, _Hmox1_−/− mean values. (b) Red blood cell counts (RBC), packed cell volume (hematocrit, Hct), blood hemoglobin concentration (Hb), and mean cell volume (MCV) analyzed in 7–11 male and female _Hmox1_−/− and Hmox1+/− littermates at 6, approximately 20, and approximately 50 weeks of age. Significant differences (P < 0.05) were observed for each parameter at all ages except for 6-week RBC and Hb values. (c) Serum iron (Fe), total iron-binding capacity (TIBC), and iron saturation analyzed in 5 Hmox1+/− and 5 _Hmox1_−/− male mice of each age group. Significant differences (P < 0.05) were observed in each parameter except for 6-week serum iron values. Iron saturation percentage is equal to 100(serum iron/TIBC). (d) Serum ferritin values analyzed in mice from c. Significant differences (P < 0.05) were observed at each age examined.
Figure 3
Iron-loading in _Hmox1_−/− tissues. (a) Kidney section from 50-week-old Hmox1+/− mouse stained with Prussian blue for detection of loosely bound ferric iron, shown at low magnification. Stainable renal iron was rarely detectable in Hmox1+/− or Hmox1+/+ mice. (b) Kidney section from 50-week-old _Hmox1_−/− mouse stained with Prussian blue. Note the intense blue positive staining in the proximal cortical tubules (arrows), which was consistently observed in _Hmox1_−/− renal tissue. (c) Liver section from 50-week-old Hmox1+/− mouse stained with Prussian blue and at low magnification. Mice containing functional Hmox1 displayed no hepatic iron deposits. (d) Liver section from 50-week-old _Hmox1_−/− mouse stained with Prussian blue, demonstrating iron-loaded Kupffer cells (arrows), as well as diffusely staining hepatocytes. Note the faint-staining regenerative nodule indicative of injury (asterisk). All _Hmox1_−/− mice displayed hepatic iron-loading by 50 weeks of age, varying in severity. (e) High-magnification view of liver section from 50-week-old Hmox1+/− mouse stained with Prussian blue, displaying no detectable iron in hepatocytes. (f) High-magnification view of liver section from 50-week-old _Hmox1_−/− mouse stained with Prussian blue, indicating iron-positive granules in hepatocytes (arrowheads), and intensely staining Kupffer cells (arrows). (All magnification bars = 100 μm.)
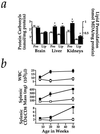
Figure 4
Markers of stress and inflammation in _Hmox1_−/− mice. (a) Protein carbonyls were measured by the method utilizing 2,4-dinitrophenylhydrazine in supernatant harvested from Hmox1+/− and _Hmox1_−/− brain, liver, or kidneys. Estimates of lipid peroxidation were obtained by malondialdehyde (MDA) measurements of tissue homogenates. Data represent mean ± SEM from duplicate or triplicate determinations from each of 4 mice of each genotype for brain samples, and each of 6–8 mice of each genotype for liver and kidney samples. Open bars represent Hmox1+/− values, while closed bars represent _Hmox1_−/− values. ∗, _Hmox1_−/− liver and kidneys, which consistently showed iron loading, had significantly greater oxidative damage than those from Hmox1+/− animals (P < 0.05). Note that _Hmox1_−/− brains, which had no iron deposition, showed no evidence of enhanced oxidative damage. (b) Average white blood cell counts (WBC), splenic mass, and splenic CD4+:CD8+ T-cell ratios analyzed from 7–11 mice of each age group for WBC, and 4–7 mice of each age group for splenic mass and T-cell ratios, at 6, approximately 20, and approximately 50 weeks of age. Significant differences (P < 0.05) were observed for each parameter at each age examined. □, Hmox1+/− values; ▪, _Hmox1_−/− values.
Figure 5
Hepatic and renal pathology of _Hmox1_−/− animals. (a) Liver section from 50-week-old Hmox1+/− mouse, stained with hematoxylin and eosin and shown at low magnification. (b) Liver section from 50-week-old _Hmox1_−/− mouse, with typical pattern of lesions. Vascular lesions are indicated by arrows. (c) High-magnification view of typical periportal fibrosis and inflammation from a 50-week-old _Hmox1_−/− mouse. This section was stained with Masson’s trichrome reagent, which reacts with collagen, an indicator of fibrosis, as bright blue. (d) High-magnification view of hepatic vascular lesion shown in b stained with hematoxylin and eosin. Note the proliferation of smooth muscle, the infiltration of neutrophils and lymphocytes into the vessel wall, and the monocytes adhered to the inner vessel wall (arrows). (e) High-magnification view of hepatic vascular lesion from a 50-week-old _Hmox1_−/− mouse. This section was stained for ferric iron (blue). Note the iron-laden monocytes adhering to the vessel wall (arrows), as well as the iron-laden vascular and connective tissue (arrowheads). (f) High-magnification view of kidney section of 75-week-old Hmox1+/− mouse, stained with hematoxylin and eosin, displaying normal glomerular morphology (arrows). (g) Kidney section of 75-week-old _Hmox1_−/− mouse displaying severely damaged glomeruli with membranous proliferation, lobularity, crescent formation, and sclerosis (arrows). (All magnification bars = 100 μm.)
Similar articles
- Reduced stress defense in heme oxygenase 1-deficient cells.
Poss KD, Tonegawa S. Poss KD, et al. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10925-30. doi: 10.1073/pnas.94.20.10925. Proc Natl Acad Sci U S A. 1997. PMID: 9380736 Free PMC article. - Altered Expression of Heme Oxygenase 2 in Heme Oxygenase 1-deficient Mouse Embryos.
Rana M, Bajaj D, Choubey P, Jain S, Basu-Modak S. Rana M, et al. J Histochem Cytochem. 2023 Aug;71(8):431-450. doi: 10.1369/00221554231189310. Epub 2023 Jul 22. J Histochem Cytochem. 2023. PMID: 37480265 Free PMC article. - Infused wild-type macrophages reside and self-renew in the liver to rescue the hemolysis and anemia of _Hmox1_-deficient mice.
Kim KS, Zhang DL, Kovtunovych G, Ghosh MC, Ollivierre H, Eckhaus MA, Rouault TA. Kim KS, et al. Blood Adv. 2018 Oct 23;2(20):2732-2743. doi: 10.1182/bloodadvances.2018019737. Blood Adv. 2018. PMID: 30337301 Free PMC article. - The clinical relevance of heme detoxification by the macrophage heme oxygenase system.
Yeudall S, Upchurch CM, Leitinger N. Yeudall S, et al. Front Immunol. 2024 Mar 22;15:1379967. doi: 10.3389/fimmu.2024.1379967. eCollection 2024. Front Immunol. 2024. PMID: 38585264 Free PMC article. Review. - Protective role of heme oxygenase in the blood vessel wall during atherogenesis.
Hoekstra KA, Godin DV, Cheng KM. Hoekstra KA, et al. Biochem Cell Biol. 2004 Jun;82(3):351-9. doi: 10.1139/o04-006. Biochem Cell Biol. 2004. PMID: 15181468 Review.
Cited by
- Expression patterns of iron regulatory proteins after intense light exposure in a cone-dominated retina.
Maurya M, Nag TC, Kumar P, Roy TS. Maurya M, et al. Mol Cell Biochem. 2021 Sep;476(9):3483-3495. doi: 10.1007/s11010-021-04175-5. Epub 2021 May 13. Mol Cell Biochem. 2021. PMID: 33983563 - Age-Related Macular Degeneration and Mitochondria-Associated Autoantibodies: A Review of the Specific Pathogenesis and Therapeutic Strategies.
Qu S, Lin H, Pfeiffer N, Grus FH. Qu S, et al. Int J Mol Sci. 2024 Jan 28;25(3):1624. doi: 10.3390/ijms25031624. Int J Mol Sci. 2024. PMID: 38338904 Free PMC article. Review. - Macrophages and iron trafficking at the birth and death of red cells.
Korolnek T, Hamza I. Korolnek T, et al. Blood. 2015 May 7;125(19):2893-7. doi: 10.1182/blood-2014-12-567776. Epub 2015 Mar 16. Blood. 2015. PMID: 25778532 Free PMC article. Review. - Heme oxygenase-1 deficiency promotes the development of necrotizing enterocolitis-like intestinal injury in a newborn mouse model.
Schulz S, Wong RJ, Jang KY, Kalish F, Chisholm KM, Zhao H, Vreman HJ, Sylvester KG, Stevenson DK. Schulz S, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Jun 1;304(11):G991-G1001. doi: 10.1152/ajpgi.00363.2012. Epub 2013 Apr 11. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23578787 Free PMC article. - Carbon Monoxide Exposure Does Not Improve The In Vitro Fertilization Rate of Oocytes Obtained from Heterozygous Hmox1 Knockout Mice.
Romanelli F, Zenclussen ML, Zenclussen AC, Meyer N. Romanelli F, et al. Int J Fertil Steril. 2023 Nov 7;18(1):76-80. doi: 10.22074/ijfs.2023.1982726.1411. Int J Fertil Steril. 2023. PMID: 38041463 Free PMC article.
References
- Means R T, Krantz S B. Blood. 1992;80:1639–1647. - PubMed
- Halliwell B, Gutteridge J M C. Methods Enzymol. 1990;186:1–85. - PubMed
- Bacon B R, Tavill A S. In: Hepatology. A Textbook of Liver Disease. Zakim D, Boyer T D, editors. Philadelphia: Saunders; 1996. pp. 1439–1472.
- Bothwell T H, Charlton R W, Motulsky A G. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 2237–2269.
- Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials