Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil - PubMed (original) (raw)
Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil
D A Fidock et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Increasing resistance of Plasmodium falciparum malaria parasites to chloroquine and the dihydrofolate reductase (DHFR) inhibitors pyrimethamine and cycloguanil have sparked renewed interest in the antimalarial drugs WR99210 and proguanil, the cycloguanil precursor. To investigate suggestions that WR99210 and proguanil act against a target other than the reductase moiety of the P. falciparum bifunctional DHFR-thymidylate synthase enzyme, we have transformed P. falciparum with a variant form of human DHFR selectable by methotrexate. Human DHFR was found to fully negate the antiparasitic effect of WR99210, thus demonstrating that the only significant action of WR99210 is against parasite DHFR. Although the human enzyme also resulted in greater resistance to cycloguanil, no decrease was found in the level of susceptibility of transformed parasites to proguanil, thus providing evidence of intrinsic activity of this parent compound against a target other than DHFR. The transformation system described here has the advantage that P. falciparum drug-resistant lines are uniformly sensitive to methotrexate and will complement transformation with existing pyrimethamine-resistance markers in functional studies of P. falciparum genes. This system also provides an approach for screening and identifying novel DHFR inhibitors that will be important in combined chemotherapeutic formulations against malaria.
Figures
Figure 2
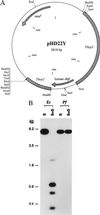
(A) Map of the P. falciparum transfection vector pHD22Y expressing the highly MTX-resistant human L22Y dhfr allele under regulatory control of the P. falciparum elements 5′-hrp3 and 3′-hrp2 (28). (B) Detection of pHD22Y DNA replicated as episomes in transfected P. falciparum. T1+MTX genomic DNA (prepared 2 months posttransfection) and DNA from a representative plasmid rescued from T1+MTX were restricted with _Sca_I (S; which cuts once in the vector) or _Sca_I + _Dpn_I (S+D; with over a dozen recognition sites) and hybridized with a probe corresponding to the pBluescriptII SK(+) vector backbone. Although _P. falciparum_-replicated episomes were resistant to _Dpn_I digestion, digestion of _E. coli_-replicated plasmids resulted in detection of bands of the expected sizes 2.3, 1.0, 0.7, and 0.3 kb (the largest being fainter as the majority of this fragment contains 5′-hrp3 sequences).
Figure 1
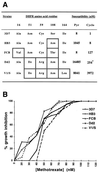
(A) Pyrimethamine and cycloguanil susceptibilities of P. falciparum lines containing different point mutations in the DHFR sequence (ref. and unpublished data). ∗, Value reflects folate and _p_-aminobenzoic acid (PABA) antagonism of the effect of cycloguanil on Dd2 (4). (B) Growth inhibition curves of the five different parasite lines as a function of MTX concentration.
Figure 3
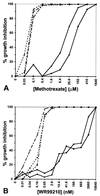
Inhibition levels of transfected versus control parasites as a function of (A) MTX and (B) WR99210 concentration, shown in logarithmic scale (log4). These assays were performed on three separate occasions, with similar results. --×--, FCB; —•—, T1+MTX; —○–-, T1-MTX; —▪—, T2+MTX; —□–-, T2-MTX. Lines T1+MTX and T2+MTX had been maintained 65 days under MTX pressure whereas lines T1-MTX and T2-MTX were grown for 39 days under pressure and then a further 26 days (13 generations) without MTX.
Figure 4
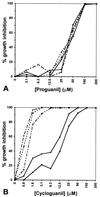
Effect of (A) proguanil and (B) cycloguanil on human DHFR-transfected versus nontransfected control parasites. Inhibition levels are shown as a function of drug concentration, shown in logarithmic scale (log2). These results were confirmed on three separate occasions. --×--, FCB; —•—, T1+MTX; —○–-, T1-MTX; —▪—, T2+MTX; —□–-, T2-MTX. The inhibitory concentrations required for cycloguanil on the parental strain FCB closely matched those recorded for this same strain by Childs and Lambros (10), though the concentrations are 10-fold higher than those determined by Peterson et al. (4). Sequencing of the dhfr loci confirmed identity between FCB used in this study and that previously described (4).
Similar articles
- Antifolate resistance due to new and known Plasmodium falciparum dihydrofolate reductase mutations expressed in yeast.
Cortese JF, Plowe CV. Cortese JF, et al. Mol Biochem Parasitol. 1998 Aug 1;94(2):205-14. doi: 10.1016/s0166-6851(98)00075-9. Mol Biochem Parasitol. 1998. PMID: 9747971 - Pyrimethamine and WR99210 exert opposing selection on dihydrofolate reductase from Plasmodium vivax.
Hastings MD, Sibley CH. Hastings MD, et al. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13137-41. doi: 10.1073/pnas.182295999. Epub 2002 Aug 27. Proc Natl Acad Sci U S A. 2002. PMID: 12198181 Free PMC article. - The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum.
Le Bras J, Durand R. Le Bras J, et al. Fundam Clin Pharmacol. 2003 Apr;17(2):147-53. doi: 10.1046/j.1472-8206.2003.00164.x. Fundam Clin Pharmacol. 2003. PMID: 12667224 Review. - The dihydrofolate reductase-thymidylate synthetase gene in the drug resistance of malaria parasites.
Hyde JE. Hyde JE. Pharmacol Ther. 1990;48(1):45-59. doi: 10.1016/0163-7258(90)90017-v. Pharmacol Ther. 1990. PMID: 2274577 Review.
Cited by
- Generation of a new DiCre expressing parasite strain for functional characterization of Plasmodium falciparum genes in blood stages.
Bansal A, Sharma M, Choudhury H. Bansal A, et al. Sci Rep. 2024 Oct 15;14(1):24076. doi: 10.1038/s41598-024-75657-x. Sci Rep. 2024. PMID: 39402380 Free PMC article. - Identification of the drug/metabolite transporter 1 as a marker of quinine resistance in a NF54×Cam3.II P. falciparum genetic cross.
Kanai M, Mok S, Yeo T, Shears MJ, Ross LS, Jeon JH, Narwal S, Haile MT, Tripathi AK, Mlambo G, Kim J, Gil-Iturbe E, Okombo J, Fairhurst KJ, Bloxham T, Bridgford JL, Sheth T, Ward KE, Park H, Rozenberg FD, Quick M, Mancia F, Lee MCS, Small-Saunders JL, Uhlemann AC, Sinnis P, Fidock DA. Kanai M, et al. bioRxiv [Preprint]. 2024 Oct 1:2024.09.27.615529. doi: 10.1101/2024.09.27.615529. bioRxiv. 2024. PMID: 39386571 Free PMC article. Preprint. - Application of optical tweezer technology reveals that PfEBA and PfRH ligands, not PfMSP1, play a central role in Plasmodium falciparum merozoite-erythrocyte attachment.
Kals E, Kals M, Lees RA, Introini V, Kemp A, Silvester E, Collins CR, Umrekar T, Kotar J, Cicuta P, Rayner JC. Kals E, et al. PLoS Pathog. 2024 Sep 23;20(9):e1012041. doi: 10.1371/journal.ppat.1012041. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39312588 Free PMC article. - Malaria parasites require a divergent heme oxygenase for apicoplast gene expression and biogenesis.
Blackwell AM, Jami-Alahmadi Y, Nasamu AS, Kudo S, Senoo A, Slam C, Tsumoto K, Wohlschlegel JA, Caaveiro JMM, Goldberg DE, Sigala PA. Blackwell AM, et al. bioRxiv [Preprint]. 2024 Oct 13:2024.05.30.596652. doi: 10.1101/2024.05.30.596652. bioRxiv. 2024. PMID: 38853871 Free PMC article. Updated. Preprint. - APEX2-based proximity proteomic analysis identifies candidate interactors for Plasmodium falciparum knob-associated histidine-rich protein in infected erythrocytes.
Charneau S, de Oliveira LS, Zenonos Z, Hopp CS, Bastos IMD, Loew D, Lombard B, Pandolfo Silveira A, de Carvalho Nardeli Basílio Lobo G, Bao SN, Grellier P, Rayner JC. Charneau S, et al. Sci Rep. 2024 May 16;14(1):11242. doi: 10.1038/s41598-024-61295-w. Sci Rep. 2024. PMID: 38755230 Free PMC article.
References
- Zolg J W, Plitt J R, Chen G X, Palmer S. Mol Biochem Parasitol. 1989;36:253–262. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources